Flatworms, the masters of regeneration – but nothing can happen without stem cells
Planarians are known as masters of regeneration: they can re-build any part of their bodies after amputation. This ability relies on a large number of pluripotent stem cells. To further investigate the mechanisms that enable planarians to maintain their stem cell pool over generations, scientists have now established a method for analysing the composition of planarian stem cells and the turnover of their proteins. They discovered a protein that is not only required for the maintenance of the stem cell pool in planarians, but might also be active in the pluripotent stem cells of mammals.
Of earthworms and flatworms
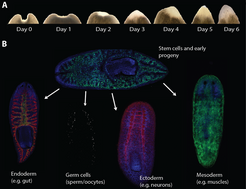
Everyone knows the myth about earthworms: if you cut them in half, you get two worms. Nothing could be further from the truth, alas. However, if the earthworm is replaced by a flatworm, the two parts can survive these childish experiments. What’s more, be it skin, intestine or brain, the body part lost through cutting will simply grow again in a matter of days. The creatures involved here are planarians [1], a class of flatworms that are so flat that they need neither lungs nor a heart to take in and distribute oxygen in their bodies. So simple and yet so ingenious? It would appear so. Regeneration studies involving these animals have shown that a dismembered planarian can generate several hundred tiny animals, hence they could “almost be called immortal under the edge of a knife” (Dalyell, 1814). The astonishing aspect here is that both the blueprint and construction material for the regeneration process must be contained in each of the fragments: a small piece of tail, for example, becomes a complete worm under the animal’s own strength and using existing resources.
Not the preserve of youth: pluripotency also available in adults
So where do the components needed to rebuild the cellular structures come from? In their search for the answer to this question, scientists have a population of small cells in their sights, namely the approximately five-micrometre-long neoblasts. These cells are found almost everywhere in the planarian body and behave like stem cells: they divide, renew and can form the different cell types that have been lost as a result of amputation (Fig. 1). When the planarian loses a body part or discards its tail for reproduction, the neoblasts are reactivated and migrate to the wound. They divide there and their offspring form a blastema, in which – as a result of interplay between various extra- and intra-cellular factors – important differentiation and patterning processes take place. Thanks to these processes, in turn, complex structures like the brain are formed. If the neoblasts are eliminated through radiation, for example, the planarian loses its ability to regenerate and dies within a few weeks. The fact that, following transplantation into an irradiated, neoblast-free worm, a single neoblast can produce all cell types and enable the host worm to regain its ability to regenerate shows that at least some neoblasts are pluripotent [2]. In healthy mammals, pluripotency, that is the ability of one cell to produce any given cell type found in an organism, e.g. muscle, nerve or pancreas cells, only arises in the early embryonic stage. Therefore, stable pluripotency in the adult organism is something special but not impossible as long as mechanisms exist for conserving this characteristic – as is clearly the case with the planarians.
An in-vivo Petri dish for pluripotent stem cells
The preservation of pluripotency has been an important topic in stem cell research for years, and has mostly been examined up to now using isolated embryonic stem cells. Important transcription factors that can induce and preserve pluripotency were discovered in the course of this research. So what can planarians contribute to the current research if their stem cells cannot be cultivated and reproduced outside of the body? This is precisely where the strength of the planarians as a model system in stem cell research lies: the combination they can offer of a natural extracellular environment and pluripotent stem cells. Whereas cultivated stem cells are normally taken out of their natural environment and all important interactions with neighbouring cells and freely moving molecules are interrupted as a result, the stem cells in planarians can be observed and manipulated under normal conditions in vivo. Therefore, planarians are of interest as “in-vivo Petri dishes” for stem cells, in which not only their mechanisms for preserving pluripotency can be studied, but also their regulation and contribution to regeneration.
A venerable old worm meets ultra-modern next-generation technologies
Although planarians have been renowned as masters of regeneration and research objects for generations, they have undergone a genuine explosion in research interest in recent years. In particular, the possibility of switching off specific genes through RNA interference (RNAi) and the availability of the genome sequence of Schmidtea mediterranea, a planarian species which is especially good at regenerating itself, have contributed to this surge in interest. With the development of modern sequencing procedures, that is ‘next generation sequencing’, gene expression profiles that provide information about the specific genes activated in particular cells or tissues at particular points in time can now be produced on a large scale. Hence, it is possible to examine which messenger RNAs (mRNAs) are produced that act as molecular templates for the production of proteins. For example, hundreds of these mRNAs are produced after the amputation of a worm’s head and their proteins provide potential regulators of the regeneration process [3; 4]. However, the real work only starts here: the extent to which the presence of a particular mRNA also reflects the volume of protein that is active in the cell remains to be determined. It is mainly the proteins in the form of enzymes, signalling molecules and structural elements, and not their mRNAs, that ultimately control the majority of cellular processes. In addition, their production using mRNA templates and their lifetime are precisely regulated processes and the frequency with which an mRNA arises cannot provide any information about these processes. The time has come, therefore, to develop experimental approaches for planarians that extend beyond gene expression analysis and lend greater significance to the subsequent regulatory processes.
No fingers but a ‘protein fingerprint’
The development of quantifiable protein measurement methods is an important step in this direction that would enable the identification of the proteins that actually arise in the planarians’ stem cells and the volumes in which they occur. Combined with the marking of proteins, quantitative mass spectrometry, which enables the identification of thousands of protein fragments based on their mass, provides a starting point here. For example, proteins can be marked through metabolism by inserting ‘heavy’ amino acids – that is the building blocks of the proteins – into the growing protein during protein synthesis (Stable Isotope Labelling by Amino Acids in Cell Culture: SILAC). The natural amino acid lysine, for example, contains six carbon atoms, each of which has six neutrons and six protons (12C6 lysine). If this natural lysine is replaced with a heavy isotope, that is a lysine whose carbon atoms contain seven neutrons and six protons (13C6 lysine), the corresponding protein becomes heavier and differs from the ‘light’ protein in the mass spectrum. This enables the direct comparison of the abundance of a particular protein present in marked and non-marked samples.
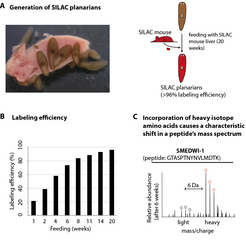
A natural characteristic of the planarians was exploited to enable the application of the SILAC method to the worms: their predilection for mashed calf’s liver, which is commonly fed to them under laboratory conditions. To mark their proteins with heavy amino acids, the worms were presented with liver from SILAC mice [5], which they consumed with relish and without suffering any side effects. After around 20 weeks, all of the planarian proteins were marked with the heavy amino acid 13 C6 lysine, therefore they were ‘converted’ at least once (Fig. 2).
Consequently, with the help of the SILAC method, the lifetime of a protein can be estimated and protein volumes can be compared at different times and in different tissues. To specifically identify the proteins that accumulate in the stem cells of the planarians, the frequency of the proteins in stem cells and in already specialised cells was measured and compared. Of the approximately 4,000 proteins identified, around one tenth had accumulated in the stem cells and were therefore responsible for their ‘protein fingerprint’, that is the stem cell proteome. They included numerous proteins that arise in a similar form in other organisms and their stem cells, and had been linked with various processes, for example the control of chromatin structure, gene expression and metabolism. An astonishing proportion of these proteins - over one third - could have something to do with the regulation of protein production itself, and this would suggest that the volume of mRNA does not actually reflect the number of the associated proteins [6].
A nuclear protein with potential
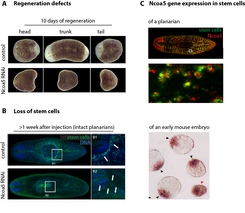
While there are many differences between the stem cells of mice and those of planarians, they also have things in common. A nuclear protein was actually found in the SILAC proteome that had not been directly linked with stem cells before. This protein, which is known as nuclear receptor co-activator 5 (Ncoa5), belongs to the family of proteins that can control the activation of genes and therefore convert the orders that a cell receives from the extracellular environment into reactions. A change in the gene expression can have serious consequences for the cell and, for example, make a specialised muscle or nerve cell out of a pluripotent cell.
The targeted deactivation of Ncoa5 with RNAi actually caused serious regeneration defects and the planarians lost all of their stem cells in a matter of a few weeks. Interestingly, the activation of Ncoa5 expression in the pluripotent cells of the early mouse embryo could be detected in experiments on mouse embryos (Fig. 3) [6]. Further research is currently being carried out to determine the mechanism whereby Ncoa5 contributes to the preservation of the presence of stem cells in planarians and whether its counterpart in the mouse is also necessary to for the preservation of pluripotency.
Despite the rapid progress being made, the technologies for researching the planarian stem cells and their self-preservation mechanisms are still in their infancy. Flatworm science can look forward to more technical innovations until we finally understand how planarians manage to preserve their pluripotent stem cell pool over generations and thereby conserve their plasticity. One such innovation will involve the development of genetically modified animals, with which it will be possible to manipulate relevant proteins in a tissue and time-specific manner, and to observe the stem cells under the microscope during the development of a regenerating head. Despite the obvious major differences, the small similarities already suggest that it is possible for us to learn from the flatworm: how regeneration is done when you have those ‘jacks-of-all-trades’ stem cells.
For bibliography, please check the Yearbook version of this article here.














