An evolutionary history of immune receptor diversity
How the immune system learned to distinguish self from non-self
Our powerful immune system is capable of recognizing almost any chemical structure. But how does this astounding discriminatory power come about, and why do immune cells attack only infected but not healthy tissues? About 50 years ago, scientists discovered how antibodies and cellular receptors known as T cell receptors (TCRs) are assembled. Much like selecting numbers in a lottery, fragments of genes are selected and fused together in a combinatorial fashion to produce functional antigen receptor. In this way, billions of combinations can be made from small sets of genetic elements. However, it has remained unclear how, at the inception of this so-called somatic recombination mechanism, immunologically beneficial expanded receptor diversity was traded against the risk of inadvertent self-recognition. Scientists at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg now offer a solution to this conundrum. They discovered a surprisingly simple molecular mechanism that initially constrained the sequence diversity of antigen receptors and charted its evolutionary modulation across 500 million years of vertebrate evolution. Initial constraints may have provided a critical time window during which to evolve specialized self-tolerance mechanisms as a prerequisite of steadily increasing receptor diversity without the risk of intolerable collateral damage.
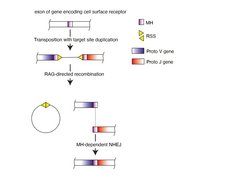
Immune receptors are expressed by lymphocytes, a white blood cell type that is generated in primary immune organs, such as the thymus and the bone marrow. Before lymphocytes are licensed to exit from these organs, they are subjected to a rigorous selection process that removes or inactivates all lymphocytes that express potentially self-reactive receptors. Failure to properly execute this tolerization step likely results in autoimmunity, or in ‘horror autotoxicus’, a scenario described for the first time by Paul Ehrlich more than 100 years ago. When Karl Landsteiner discovered in the 1920s that our body can raise antibodies against almost any chemical structure and to distinguish among them with astounding precision, highlighting the enigma surrounding the inner workings of the immune system. How, scientists asked, is it possible to encode so many specificities when the genome of animals simply doesn´t have the required? Many decades later, Susumu Tonegawa’s research provided a solution to this riddle, by demonstrating that antigen receptor genes are stitched together in an intricate recombination mechanism from smaller elements, very much like hundreds of millions of combinations can be constructed from a small set of numbers in the popular lotteries. Yet, the discovery of this dedicated recombination mechanism raised the question of its evolutionary origin, for which Tonegawa proposed the ‘transposon-split-gene’ hypothesis about 50 years ago.
Transposon splits receptor-encoding gene
In this scenario, the insertion of a transposon (also known as ‘jumping gene’) split an exon of a cell surface receptor-encoding gene, to create proto-variable (V) and proto-joining (J) segments that together encode the antigen binding surface of antigen receptors. The split gene could be reassembled into a functional exon, because the tandem inverted repeats flanking the inserted sequence served as recombination signal sequences guiding a recombinase to create double-stranded DNA breaks and to excise the inserted sequence; the two free ends would be joined together by the general non-homologous end joining repair pathway. To this day, a major problem remained: As transposition and the associated emergence of the split V-J gene in ancient vertebrates is a chance event, it appeared unlikely that the appropriate quality control mechanisms pre-existed that could be harnessed to suppress potentially harmful self-reactivity emanating from somatically assembled receptors encoding somewhat unpredictable antigen binding sites.
Against the backdrop of this conundrum, researchers at the Max Planck Institute of Immunobiology and Epigenetics decided to take a close look at the molecular consequences of transposon insertion. It was known from work of others that when a transposon inserts into DNA, this results in a tandem duplication of a short stretch of nucleotides flanking the foreign DNA. Could it be, they asked, that this identical ‘microhomology’ region determined the repair process to limit junctional diversity at the sealed breakpoint?
Microhomology sequences offer the possibility for the development of self-tolerance mechanisms
To test this idea, the scientists examined two predictions of their hypothesis. “Among individuals of evolutionarily older species, we should expect a greater fraction of shared sequences if the original microhomology sequences still influence the outcome of the recombination process”, explains Thomas Boehm, director of the Department of Developmental Immunology. Orlando Giorgetti, project leader in the department and first author of the study adds: “A second prediction is that, with evolutionary time, the microhomology should become less and less important, and hence the diversity of antigen receptor repertoires should steadily increase“. To the delight of the scientists, both predictions were found to be true as a result of a wide-ranging analysis of antigen receptor repertoires of more than 300 species of vertebrates of all major clades. In their current work, they focused on the T cell receptor a chain gene, which belongs to the V-J class of AgRs, reminiscent of the presumptive primordial AgR gene. It encodes one of the chains of the canonical heterodimeric αβTCR22, and is a near-universal component of adaptive immune systems of jawed vertebrates.
The new results indicate that engaging the mechanistic characteristics of non-homologous end joining repair for the V-J recombination process minimized the degree of uncertainty arising from junctional diversity. Maintenance of microhomologies derived from the target-site duplication – at least initially – may have constrained junctional diversity and provided a critical time window during which to evolve specialized self-tolerance mechanisms as a prerequisite of increasing receptor diversity without the risk of intolerable collateral damage. In future work, the authors aim at reconstructing the initial components of the tolerance machinery in order to illuminate the evolutionary forces that shape the human immune system.
TB/OG
Teaser image by Dylan Nolte on Unsplash











