Max Planck Innovation awards license for actin marker LifeAct
Max Planck Innovation and ibidi sign license agreement for cytoskeleton research
Max Planck Innovation GmbH, the technology transfer organization of the Max Planck Society, has awarded an exclusive license for LifeAct to ibidi GmbH, a provider of cell analysis products, located in Martinsried near Munich. The novel peptide allows for actin, an important protein, to be made visible in living cells without disturbing the actin dependent processes. Therefore, cells as well as the development of various diseases can be better researched.

All higher cells depend on their cytoskeleton. This extremely flexible and highly dynamic structure gives the cells its shape, but is also an essential component in cell division as well as other fundamental processes of life. As a structural protein, actin is a component of the cytoskeleton, which can form elongated filaments that are used, among other things, as supply routes for molecular loads within the cell. Thereby, actin plays an important role in a wide array of functions such as in the development of organs and the movement of cells. Defects in the cytoskeleton are associated with various diseases such as polycystic kidney diseases and invasive tumors. Thus, the study of the cytoskeleton and in particular the study of actin is a priority in the biomedical research.
In the past, markers were available that allowed visualization of actin, but also interfered with its activity. Whereas the actin marker LifeAct, now licensed by ibidi, attaches itself to the structural protein without limiting its function. LifeAct, only 17 amino-acids long, is linked to a fluorescent dye, which makes it possible to follow the development and movement in living cells. The marker has already been used successfully in yeast cells, kidney cells, white blood cells, neuron and a variety of other cell types and tissues. A research team led by the biologists Dr. Roland Wedlich-Söldner and Dr. Michael Sixt from the Max Planck Institute of Biochemistry developed the marker. In future, ibidi will offer the peptide in various products for biological analysis. Even pathological processes where actin is involved could be decoded.
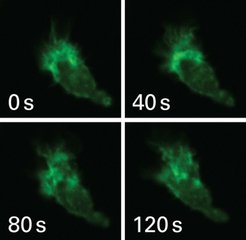
"LifeAct fits perfectly into our product portfolio with cell microscopy, immunofluorescence and cell based assays" emphasizes Dr. Valentin Kahl, founder and CEO of ibidi. "With the help of this marker, our customers can visualize and quantify the movement of cells. The new technology will be of great benefit, especially in studies of angiogenesis (the formation of blood vessels), but also in oncology, neurology, and immunology." Moreover, Dr. Mareike Göritz, patent and license manager at Max Planck Innovation, sees a great advantage in the proximity of Max Planck Institutes of Biochemistry and Neurobiology to ibidi GmbH in Martinsried: "The mutual support and uncomplicated communication should ensure a close cooperation and hence a rapid market implementation of LifeAct." This should benefit the researchers and product developers as well as the research community. Thus, LifeAct opens unprecedented opportunities to research actin as an essential component of life’s fundamental processes.
************************
ibidi GmbH
The ibidi®GmbH, Munich, develops, produces and distributes micro-slides (µ-slides) for the functional analysis of living cells in microscopy. The µ-slides constitute a new family of analysis chambers for the evaluation of cell based assays, for example for chemotaxis, angiogenesis, and tissue repair. The µ-slide family consists of different slide types for cell culture and high-end microscopy, which are distributed through partners worldwide.
Max Planck Innovation
Max Planck Innovation markets patents and technologies to industry and advises scientists when establishing new companies based on the research of Max Planck Institutes.
Every year Max Planck Innovation evaluates about 150 inventions, of which about half lead to patent applications. Since 1979, almost 3,200 inventions were accompanied and nearly 1.900 licensing agreements were completed. Moreover, since the early nineties, nearly 90 successful spin-off companies were supervised.