MPG spin-off Thermosome starts Phase I trial
First patient dosed with lead program THE001 for the treatment of soft tissue sarcoma
Thermosome, a drug development company specializing in targeted tumor therapies, today announced that the first patient has been dosed in April 2023 in the ongoing Phase 1 trial with its lead program THE001. Additional patients will be enrolled following a 6-week monitoring period that includes two treatment cycles. With this lead candidate, the spin-off from the Max Planck Institute for Biophysical Chemistry (now Max Planck Institute for Multidisciplinary Sciences) is ideally positioned to improve conventional DOX confirmed as gold standard for the neoadjuvant treatment of locally advanced soft tissue sarcoma (LA-STS).
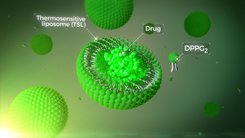
Thermosome is developing thermosensitive tumor targeting systems that can be used to release active substances enclosed in liposomes in a targeted manner by heating the tissue at the desired site of action. THE001 is a thermosensitive liposomal formulation of doxorubicin (DOX) for the treatment of anthracycline sensitive tumors with locally advanced soft tissue sarcoma (LA-STS) as the lead indication.
The trial will further enroll patients with locally advanced unresectable or metastatic soft tissue sarcoma at two clinical sites in Germany: Helios Klinikum Berlin-Buch and LMU Klinikum, Munich. THE001 will be administered at three dose levels, with three to six patients treated at each dose level (3+3 design). The primary endpoints of the Phase 1, open-label, interventional dose-escalation study are the safety and tolerability of THE001 and the determination of the maximum tolerated dose. A secondary objective is to evaluate anti-tumor activity.
"We are delighted that the first patient in our Phase I study has been dosed at LMU Klinikum," said Pascal Schweizer, co-founder and CEO/CFO of Thermosome. "Thermosome has now reached a key corporate milestone by transitioning into a clinical-stage company. We are looking forward to the results of this first-in-human study."
Dorit Di Gioia, Principal Investigator at LMU Klinikum, added: "Locally advanced soft tissue sarcoma is a disease with a very high unmet medical need, especially when it comes to novel therapeutic options for the neoadjuvant treatment of patients with localized high-grade tumors. While standard doxorubicin-based chemotherapy has only a modest effect, THE001 in combination with regional hyperthermia can achieve up to 15-fold higher local doxorubicin concentrations. This offers significant potential to enhance the treatment efficacy of the tumor and for inducing an immune response.”
Patent protected technology
Thermosome´s approach is based on a patent-protected phospholipid (DPPG2), which is incorporated into its thermosensitive liposomes. DPPG2 was invented by Hansjoerg Eibl, formerly Professor at the Max Planck Institute for Biophysical Chemistry who focused his research career on novel phospholipids and lipid-based nanocarrier systems. Florian Kirschenhofer, Start-up and Portfolio Manager at Max Planck Innovation, stated: "We are thrilled to see that research results from basic research at the Max Planck Society have been further developed by Thermosome, resulting in the dosing of the first patient with THE001 and the transition of the company into the clinical stage. This is an important milestone and we hope that this will lead to an improved treatment option for patients in the future."