Manufacturing the core engine of cell division
By modelling the kinetochore from scratch, Max Planck Institute’s researchers get a step closer to creating artificial chromosomes
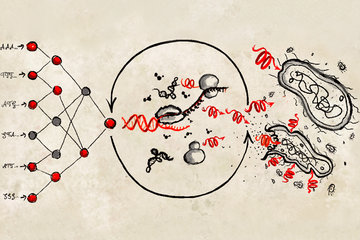
It´s a cellular process going on since one billion years, yet we are not able to replicate it, nor to fully understand it. Mitosis, the mechanism of cell division that is so important for life, involves more than 100 proteins at its core. Now, the group of Andrea Musacchio from the Max Planck Institute of Molecular Physiology in Dortmund has been able to fully reconstitute the engine of the mitosis machinery, called kinetochore. Being able to model a functioning kinetochore is the first step towards the making of artificial chromosomes, that may one day be used to restore missing functions in cells.

As a human cell begins division, its 23 chromosomes duplicate into identical copies that remain joined at a region called the centromere. Here lies the kinetochore, a complicated assembly of proteins that binds to thread-like structures, the microtubules. As mitosis progresses, the kinetochore gives green light to the microtubules to tear the DNA copies apart, towards the new forming cells. “The kinetochore is a beautiful, flawless machine: You almost never lose a chromosome in a normal cell!”, says Musacchio. “We already know the proteins that constitute it, yet important questions about how the kinetochore works are still open: How does it rebuild itself during chromosome replication? How does it bind to the microtubules? And how does it control them?”
Musacchio´s quest for answers started more than 20 years ago and has been guided by a simple motto: “Before we understand how things go wrong, we better understand why and how things work”. He therefore embarked in the mission of rebuilding the kinetochore in vitro. In 2016 he could synthesize a partial kinetochore made of 21 proteins.
In the new publication, Musacchio, his Postdoc Kai Walstein, and their colleagues at Max Planck Institute in Dortmund have been able to fully reconstruct the system: All subunits, from the ones that bind the centromere to the ones that bind the microtubules, are now present in the right numbers and stoichiometry. Scientists proved that the new system functions properly, by successfully substituting parts of the original kinetochore in the cell with artificial ones. “This is a real milestone in the reconstruction of an object that exists, unaltered, in all eukaryotic cells since more than one billion years!”, says Musacchio. This breakthrough paves the way towards the making of synthetic chromosomes carrying functions that can be replicated in organisms. “The potential for biotech applications could be huge”, he says.
In the protein factory

Max Planck scientists had to overcome a major hurdle to rebuild the kinetochore, namely to fully reconstruct the highly flexible Centromeric Protein C (CENP-C). This is an essential protein that bridges the centromeric region to the outer proteins of the kinetochore. Researchers rebuilt CENP-C by “gluing” together the two ends of it.
A highly organised laboratory, similar to a factory, is fundamental for the reconstitution of complex protein assemblies. For each protein of the kinetochore, Max Planck scientists built a production pipeline to isolate the genes, express them in insects´ cells, and collect them. “When we put them together in vitro, these proteins click-in to form the kinetochore, just like Lego pieces following the instructions”, he says. Other than the famous toys though, each kinetochore protein has a different interface and interaction with closer proteins. The group will now step up to the next level of complexity: Investigating how the kinetochore functions and interacts in the presence of microtubules and supplied energy (in the form of ATP). The project has been recently granted an ERC Synergy Grant and will be carried out by an international team comprising Musacchio’s group and researchers from Cambridge, UK, and Barcelona, Spain.