Cellular stress promotes inflammation via sphingosine-1-phosphate
The sphingolipid metabolite sphingosine-1-phosphate activates NOD receptors upon perturbation of cellular homeostasis
A team of researchers led by Stefan H.E. Kaufmann at the Max Planck Institute for Infection Biology have elucidated how changes in the cellular equilibrium alert the cell. They showed that cellular stress stimulates the formation of a sphingolipid, which is then recognized by intracellular pattern-recognizing receptors, thereby initiating inflammatory responses. The processes are similar to those that take place in chronic inflammatory diseases such as inflammatory bowel disease. These findings thus offer deeper insights into the mechanisms behind chronic inflammatory diseases and reveal targets for new treatment strategies.
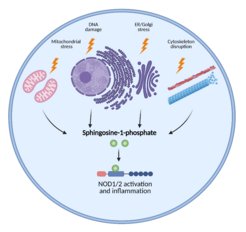
Various cellular stress responses are associated with inflammation in multiple chronic diseases. The group of Stefan H.E. Kaufmann together with their collaborators from the Friedrich-Loeffler-Institut and the Universität Hohenheim now discover that various unrelated stress inducers enhance the generation of the endogenous metabolite S1P that binds to and activates NOD1/2-mediated inflammatory responses.
NOD molecules are intracellular pattern recognition receptors (PRRs) that sense bacterial peptidoglycans to activate innate immune responses. Upon ligand binding, these PRRs through a complex signalling cascade, drive the expression of proinflammatory and antimicrobial genes. In addition to their well-known roles in bacterial sensing, the NOD receptors are also activated by various peptidoglycan-free viruses and parasites, as well as actin cytoskeleton disruption and endoplasmic reticulum (ER) stress. Whether these NOD detect other types of cellular stress and how these receptors are activated by such diverse stimuli remain elusive.
How do cells sense intracellular stress?
Gang Pei, first author of the papers, says: “To answer these questions, we took the advantage of different genetically modified cells and found that diverse stress stimuli induce NOD1/2 dependent inflammatory responses.” To elucidate the mechanism, the team performed gene expression analysis and uncovered that key enzymes involved in the sphingolipid pathway were induced by various stressors. Indeed, the production of cytosolic sphingosine-1-phosphate (S1P) was markedly increased. Following this, they employed pharmacological inhibitors targeting different steps in the sphingolipid pathway and pinpointed S1P as the critical regulator of NOD activation. Does S1P directly bind and activate NOD? Anca Dorhoi, now at the Friedrich-Loeffler-Institut, says: “We revealed that S1P specifically and directly binds to a distinct site of these NODs”. The delivery of S1P into cells triggered NOD dependent proinflammatory responses. “We thus identified a central role of S1P in triggering NOD-dependent inflammation upon perturbation of cellular homeostasis”, says collaborator Thomas Kufer from the University Hohenheim.
A new basis for novel intervention strategies for autoinflammatory disorders?
This finding unveils a hitherto unknown role of NOD in surveillance of cellular homeostasis through sensing of the cytosolic metabolite S1P. This type of NOD sensing represents an alert mechanism for infectious insult, which could also explain NOD activation by peptidoglycan-free viruses and parasites. Stefan Kaufmann, senior author of this paper, concludes: “The abundance of host sphingolipids is highly increased in inflammatory bowel disease patients and numerous NOD mutations are associated with various autoinflammatory disorders. Thus, our research provides novel insights into mechanisms of the inflammation associated with chronic inflammatory diseases and establishes that sensing of the sphingolipid S1P by the NOD receptors as a strong candidate target for novel therapeutic strategies.”