Biosensor for phenylketonuria
With the help of a new blood test patients with this disease can monitor their metabolites
The treatment of numerous diseases could be improved if the blood concentration of disease-relevant metabolites could be monitored at the point-of-care (POC), ideally even by the patient. A team of scientists led by researchers at the Max Planck Institute for Medical Research in Heidelberg and the École Polytechnique Fédérale de Lausanne has now introduced a biosensor for the accurate quantification of metabolites in small blood samples obtained from a simple finger prick. This biosensor could become an important tool for the diagnosis and management of various diseases.
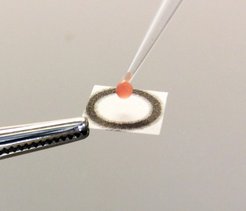
Diseases or injuries can result in dramatic changes in metabolite blood levels. For example, increased blood levels of the amino acid phenylalanine are characteristic of the genetic disorder phenylketonuria. Infants suffering from this disease need to control phenylalanine levels through dietary management to avoid irreversible brain damage. It is therefore essential to have some means of regularly monitoring phenylalanine levels in blood.
However, such monitoring currently requires blood samples to be sent to clinical laboratories with results taking several days to come back to the patient. This time-delay often complicates disease management for both phenylketonuria patients and their physicians. A team of scientists led by Kai Johnsson of the Max Planck Institute for Medical Research in Heidelberg has now introduced a simple paper-based assay that can measure concentrations of metabolites such as phenylalanine in small blood samples within minutes. The approach was validated with patient samples obtained from the Heidelberg and Lausanne University Hospitals. The work represents an important advance towards POC-monitoring of metabolites in blood.
Molecular engineering
“We introduce a fundamentally new mechanism to measure metabolites for blood analysis,” says Qiuliyang Yu, first author of the paper and scientist at the Department of Chemical Biology at the Max Planck Institute in Heidelberg. “Instead of miniaturizing available technologies for POC applications, we developed a new molecular tool,” he explains further. The tool is a light-emitting, engineered protein that changes its color in the presence of the reduced cofactor nicotinamide adenine dinucleotide, known to biochemists under its acronym NADPH. As NADPH is produced in an enzyme-catalyzed reaction specific for the metabolite of interest, analyzing the color of the emitted light reveals the metabolite concentration. Using different enzyme-catalyzed reactions, the same sensor can be used to establish quantitative POC assays for various metabolites, as shown for phenylalanine, glutamate and glucose.
How to use it?
In the case of phenylalanine, a droplet of blood is first taken from the patient through a painless finger pick. In a second step, a fraction of the blood sample is added to a reaction buffer and applied onto a paper containing the immobilized biosensor. When phenylalanine is consumed and NADPH is produced, the light emitted by the sensor changes its color from blue to red, which can be detected by a simple digital camera or a smartphone. The ratio of blue to red light is then used to calculate the concentration of phenylalanine. The whole procedure takes only ten to 15 minutes, can be done at the POC and yields accurate results when compared to standard methods used in clinical laboratories.
The accuracy and simplicity of the procedure should eventually enable patient self-testing, a goal that is pursued at the Max Planck Institute for Medical Research. “We are now looking for ways to further automatize and simplify the test” says Yu, who is excited about the idea of putting a much needed tool in the hands of patients suffering from phenylketonuria or other diseases that require the monitoring of metabolite concentrations.