Origins of Life: Worlds Meet
Life on Earth is based on nucleic acids, and recent findings have energized the study of how these emerged from the simple molecules on early Earth. Some researchers believe that amino acids and water were first delivered to Earth by asteroids or comets. Observations from Earth are informing the search for signatures of biological activity on planets beyond the Solar System.
Besides carrying genetic code, RNA also catalyses the translation of that code into proteins. This means that RNA can account for a surprising amount of the story.
The question of how life arose from the non-living has troubled humankind since the dawn of civilization, but it was not until the twentieth century that modern science started to provide answers. Building on ideas from Alexander Oparin in the 1920s, in 1953 Stanley Miller and Harold Urey cooked up a primordial soup that spawned amino acids and other organic compounds — some of the building blocks of life. Without a decisive breakthrough over the following decades, however, progress started to die down.
It took the discovery of the first planet in another Sun-like star system in 1995 to reignite interest in the conditions necessary for life1. Since then, astrophysicists, planetary scientists and biologists have been working together in what has become a golden age. Even so, our model for understanding the atmospheres of young planets relies on what we know of the early Earth, about which we still have much to learn. We also have only one example of life — cell- and DNA-based — and whether these elements are required for life, or just for one possible version of it, remains unknown.
Precursors of precursors
We will only ever understand the origins of life on early Earth when we can explain how large, information-bearing and self-replicating polymers could have emerged from the simple molecules available at the time. Some large composites are relatively easy to rationalize, such as membranes formed from partially hydrophobic molecules that grouped together, eventually shielding more delicate material, such as DNA, within. The real conundrum is how DNA itself first appeared, along with the complex way in which it works: it couples with a similar, shorter molecule known as RNA, which copies the code from DNA and uses it to piece amino acids together into proteins.

It has been proposed that RNA holds the secret behind today’s protein-based life forms. This ‘RNA world’ hypothesis gained considerable support when, in 1981, it was found that besides carrying genetic code, RNA also catalyses the translation of that code into proteins, in cell components known as ribosomes. This dual role means that RNA can account for a surprising amount of the story.
Although ‘pre-RNA world’ hypotheses, which claim that simpler self-replicating molecules preceded RNA as the genetic material, have some support2, the RNA world hypothesis currently prevails thanks to a growing body of evidence.
In 2009, ribonucleotides — precursors to DNA and the ‘letters’ of genetic code — were produced under conditions that were consistent with early Earth models3. More recently, a study that reproduced some of the conditions found in deep-sea hydrothermal vents (Fig. 1) gave a clue to another longstanding mystery. This study found that small-scale temperature gradients create ‘thermal traps’ that can concentrate nucleotides, join them together and create RNA. This RNA is then also trapped4. The use of a mathematical model in that study illustrates that we will not find all of the answers to the long, slow rise of life in the test tube.
Extra-terrestrial origins
The ingredients for life may have come from far beyond Earth. Planets form from flat clouds of dense gas and dust around young stars, known as protoplanetary disks, which contain both water and complex organic molecules. Primitive meteorites and comets also harbour these chemicals, so some scientists hope to compare the chemical compositions of each of these potential sources in the hope that this might reveal how early Earth became favourable for life. The trouble is, samples of these materials are not easy to come by.
Some meteorites even contain amino acids, and might therefore be responsible for the vital role that these molecules play in life on Earth. There is a problem, however — most amino acids are chiral (either appearing in a ‘left-handed’ form or its mirror image, the ‘right-handed’ form), and although all amino acids in proteins on Earth are left-handed, most meteorites that harbour amino acids contain an even mix of these forms. Even so, a few meteorites have been found to contain an excess of left-handed amino acids5. If an imbalance such as this were later amplified, perhaps through left-handed amino acids being used as a template in a primitive replication system, it could account for the chiral nature of proteins on Earth. And it may not be a mere coincidence that life emerged on Earth at around the time of the Late Heavy Bombardment, when asteroids would have repeatedly delivered organic molecules to Earth.
Home away from home
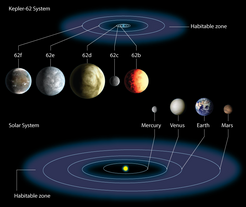
Even further afield, nearly 2,000 exoplanets have so far been spotted orbiting stars beyond the Solar System, thanks to ground based observations and NASA’s Kepler Mission. Among these, Earth-sized planets in an orbit capable of sustaining liquid water — the habitable zone (Fig. 2) — are much more common than had once been assumed6. Astronomers are trying to characterize the thermal and chemical properties of the atmosphere of each of these far flung planets by using sophisticated optics to detect the spectral changes that they make to starlight. By comparing the signals from these atmospheres with what we would expect from biology and spectroscopy, astronomers hope to be able to work out whether or not there are any signs of life.
But what, exactly, are they looking for in these atmospheres? Once again, researchers are turning to the Earth for inspiration, and in particular the geological processes that occurred at around the time that life emerged. The clues are hard to come by — rocks that were formed before 3.5 billion years ago, when life arose, are now vanishingly rare. Even so, we are beginning to piece together how the cycles of essential elements (in particular, carbon, oxygen, nitrogen, phosphorus and sulphur) gradually evolved. This knowledge will help us to look for comparable atmospheres elsewhere.
By any measure, we are still a long way from a satisfying explanation of the origins of life. What is becoming ever clearer, however, is that we will never achieve this goal by looking only in a test tube, or only at rocks. Instead, we need to develop even further the interdisciplinary approach of the past few decades, bringing researchers together from chemistry, biology and biochemistry to geology and astrophysics. When these worlds meet and the dust settles, we could find ourselves holding a realistic answer to the oldest question of all.
Life can be seen as the result of the chemical activity of proteins, yet today’s intricately folded proteins are too complex to have arisen from scratch in an abiotic environment. Researchers at the Max Planck Institute for Developmental Biology are working out how proteins might have come from shorter, primitive peptides, by showing how unrelated but structurally similar protein fragments can be joined together (Rico JA & Höcker B, Methods Enzymol. 523, 389–405; 2013).
References
1Mayor M & Queloz D, Nature 378, 355–359 (1995).
2Shapiro R in: Sullivan WT III & Baross JA (eds) Planets and Life. Cambridge University Press, pp.132–153 (2007).
3Powner MW et al., Nature 459, 239–242 (2009).
4Mast CB et al., PNAS 110, 8030–8035 (2013).
5Cronin JR & Pizzarello S, Science 275, 951–955 (1997).
6Petigura EA et al., PNAS 110, 19273–12978 (2013).













