Nature designs hard and tough materials at the nanoscale
Max Planck scientists prove that the extreme strength of biomaterials is due to an as yet unknown flaw tolerance threshold in the nanometer range
The nanoscale size of mineral particles in bone, tooth, and other biological materials may have evolved to ensure optimum strength and maximize tolerance of flaws, according to new research conducted at the Max Planck Institute for Metals Research and Austrian Academy of Sciences. While it is quite clear that the composite character of biological materials plays an important role, little is known about the role of the nanometer scale of mineral particles. The new study provides further insight into the mystery how nature produces hard and tough materials out of protein as soft as the human skin and mineral as fragile as the classroom chalk. The key finding of this research is that there exists a critical nanometer size at which the particles found in biocomposites become insensitive to flaws: They maintain strength equivalent to a perfect crystal despite inherent defects. This phenomenon also suggests that the engineering concept of stress concentration at flaws is no longer valid for nanoscale design. (Gao et al., PNAS, 2003, Vol. 100, No. 10, pp. 5597-5600.)
The science and technology of materials have set milestones of human civilization. We have mastered the art of making a wide variety of materials with many interesting mechanical properties. Some materials like ceramics, glasses and mineral crystals are hard and fragile. Others like rubbers and collagen are soft and tough. During the Bronze and Iron ages, man learned to make hard and tough materials in the form of various metals and metallic alloys. With the maturity of the field of metallurgy during the 20th century, we now understand that the hardness and toughness of metals are largely attributed to their crystalline structure which allows an important class of material defects called dislocations to move around and relieve stress concentration at crack-like flaws. One of the next big challenges for humanity is to develop hard and tough materials without dislocations, as this would open a vast new territory in materials science and technology to make novel materials with applications ranging from high temperature, light weight structures to fatigue and corrosion resistant materials. How do we achieve this objective? One suggestion would be to learn from Mother Nature; through natural evolution she has produced hard and tough non-metallic materials such as bone, dentin, shell, wood, etc. It is usually very difficult to mimic all the details of biological processes involved in the productions of biological materials. A better and more practical proposition would be to try to find out and understand the basic principles underlying biomaterial construction by clever hypothesis, modelling and experimentation. Once the basic principles are established, it may then become possible to contemplate practical materials processing techniques that mimic only the principles, without accounting for all the details, used by Mother Nature for human applications.
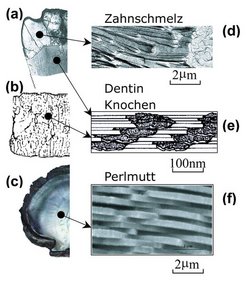
Biomaterials like bone are molecular composites of proteins and biominerals. While the stiffness of biocomposites is similar to that of the mineral, their fracture energy can be several orders of magnitude higher than the mineral. For instance, the composite shell of nacre shows a fracture strength that is 3000 times higher than its mineral constituent CaCO3. Despite the complicated hierarchical structures of biocomposites, it is most interesting to observe that the smallest building blocks in biological materials are generally on the nanometer length scale and aligned in a generic structure of mineral platelets staggered in protein matrix. Why is the nanoscale so important to biomaterials? The scientists (H. Gao, B. Ji, I. L. Jäger, E. Arzt, and P. Fratzl) of Max Planck Institute for Metals Research and Austrian Academy of Sciences and Metal Physics Institute, University of Leoben, have found that this generic nanostructure of biomaterials may be the key to high fracture strength of these materials. Their analysis indicates that the mineral crystals carry the tensile load while the protein matrix transfers the load between mineral crystals via shear. The mineral crystals have large aspect ratios in order to compensate for the large differences in stiffness between mineral and protein. In order to ensure integrity and strength of the composite structure, the mineral crystals must be able to sustain large tensile stress without fracture. The tensile strength of mineral crystals is the key to the composite strength.

Using a series of equations, the scientists illustrate that cracked mineral crystals below a critical nanometer size, estimated at 30 nanometers, have fracture strength identical to that of a perfect, defect-free crystal. They further developed a finite element method to demonstrate that the stress field near a growing crack becomes more and more uniform as the thickness of the structure decreases, eventually reaching the theoretical strength at the critical size. A particle smaller than this size becomes insensitive to crack-like flaws. The findings may explain why bone, which has particles a few nanometers thick, is stronger than shell, which has particles a few hundred nanometers thick. The scientists suggest that because materials become insensitive to flaws at this critical nanometer size, the engineering concept of stress concentration at flaws is no longer valid for nanoscale design.

