Interactions between proteins determine specificity of target genes
Protein-protein-interactions locally change the structure of the DNA and thus strengthen the sequence-specific binding of important proteins
A fundamental topic in plant development is how proteins function together in regulatory networks to coregulate the activity of specific target genes. A collaboration between researchers in the groups of George Coupland and Jijie Chai from the Max-Planck Institute for Plant Breeding Research and the University of Cologne has elucidated an elegant mechanism for how a particular protein–protein interaction cooperatively targets genes in Arabidopsis by affecting DNA conformation. The findings has wider implications for how transcription factors can achieve regulatory specificity in other developmental contexts.
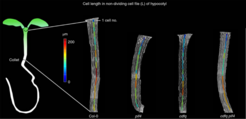
Two classes of transcription factors, PHYTOCHROME-INTERACTING FACTORs (PIFs) and CYCLING DOF FACTORs (CDFs), are involved in regulating genes that control plant growth in response to light and temperature. However, how the specificity of plant-specific DOF factors is determined has been puzzling because they are reported to recognize very short, simple DNA sequences that occur frequently in the plant genome.
In a paper, He Gao and coworkers from the laboratories of George Coupland and Jijie Chai, as well as the Genome Centre at the Max-Planck Institute for Plant Breeding Research demonstrated that in the model species Arabidopsis thaliana, two representative members of these transcription factor families, PIF4 and CDF2 act cooperatively and regulate a subset of similar genes. The authors used a powerful combination of imaging, genome-wide genomics and in vitro biochemistry to identify common target genes of PIF4 and CDF2. They then analysed in detail the binding of both proteins to one particular common target, YUCCA8, which encodes an enzyme that produces the hormone auxin in cotyledons and thereby promotes the elongation of hypocotyl cells in response to light.
Closely spaced CDF2 binding sites
Proteins regulate their target genes by recognizing and binding to specific short DNA sequences. The authors found that in the YUCCA8 gene, the simple CDF2-binding sites are very closely spaced with those of PIF4. They also identified that in vitro, PIF4 and CDF2 physically interact with each other and PIF4 increases the ability of CDF2 to bind to YUCCA8 DNA. Similarly, in plants in which PIF4 is inactivated, binding of CDF2 to its target genes, including YUCCA8, was greatly reduced in vivo. In addition to recognizing a particular DNA sequence motif, the strength and specificity with which regulatory proteins can bind their target sequences is also determined by the local conformation of the DNA at the target site, which determines how accessible the binding sequence is for protein binding.
The authors carried out a series of experiments that involved mutating the DNA-binding sites of PIF4 and CDF2 in YUCCA8 and confirming in vitro which parts of each protein interact with each other and they proposed a mechanism to explain how PIF4 and CDF2 can cooperatively activate the expression of YUCCA8. They demonstrated that four PIF4 proteins associate as a complex consisting of two PIF4 dimers and when this complex binds to its DNA target sites, it locally alters the conformation of DNA. The interaction of PIF4 with CDF2 then enhances the binding of CDF2 at its binding sites, which are located adjacent to the PIF4-binding sites.
In future, these studies can be extended to determine whether the analysis of PIF4 and CDF2 cooperation at YUCCA8 provides a general model for how both proteins regulate other gene targets, and to study their cooperation in vivo during hypocotyl elongation. An important aspect of the study is the elucidation of a specific mechanism that explains how protein–protein interactions can enhance sequence-specific DNA binding of an important class of plant-specific protein by altering local DNA shape to activate gene expression in a specific developmental context.












