A copying machine for genes
Nothing works with incomprehensible code – not even a cell. Patrick Cramer is carrying out research on the enzyme that transcribes the DNA code to enable a protein to be synthesized from a gene. To do so, he relies on high-resolution microscopes and artificial intelligence.

Text: Andreas Lorenz-Meyer
Showtime! The movie zooms right into a cell nucleus. Like a screw, the DNA double helix propels into the active centre of the RNA polymerase. “There, it is unwound, and one of the two DNA strands then serves as a template for synthesizing the messenger RNA molecule,” explains Patrick Cramer, Director at the Max Planck Institute for Multidisciplinary Sciences in Göttingen. On Cramer’s screen, the RNA product emerges as a single strand from the body of the polymerase enzyme. The RNA later serves as a construction plan for a new protein.
In technical language, this process of copying DNA into RNA is called gene transcription. “It is the process that brings our genes to life,” says Cramer. It’s no wonder that the sole function of a large fraction of our proteins is to enable and regulate transcription – about 1800 in total. And one of these players dwarfs all the others: the enzyme RNA polymerase II, which is responsible for producing messenger RNA. “Our central copying machine,” is what Cramer calls this enzyme made up of several subunits. When he tells us that the enzyme develops a force of 20 trillionths of a newton and incorporates 3000 building blocks per minute into RNA, the 54-year-old molecular biologist’s admiration for the enzyme’s capabilities is palpable. Even after years of research, he has lost none of his enthusiasm for the fascinating processes that underlie cellular life.

Cramer’s scientific career took him to Stanford University in California, where he was a postdoc from 1999 to 2001. It was during that time that Cramer became the first to decipher the complicated spatial structure of RNA polymerase II. His mentor at Stanford was Roger Kornberg, who later won the Nobel Prize for Chemistry for elucidating the molecular basis of transcription. The work at Stanford provided researchers with a first glimpse of the inner workings of the polymerase machine. In Cramer’s office at Göttingen, there is a model of the enzyme that resembles a colourful coral. Among other things, it shows the cleft in the molecule where DNA is transcribed into RNA.
The research work back then at Stanford was the starting point for decoding the entire transcription process: this enabled many questions to be answered – for example, how RNA polymerase is directed to the start site of a gene. Or why the enzyme constantly takes breaks when it slides along DNA: “These pauses are necessary because there is sometimes grit in the transcription gear, in which case, helper molecules, known as elongation factors, rush in. They remove the obstacles – and transcription can continue,” Cramer explains. To decipher the process further, the researchers have had to develop several different experimental and computer-based methods over the years.
Twenty years on, transcription research is now entering its next phase. “So far, we have reconstituted and studied the transcription process on single genes outside the cell in the test tube. Now we want to analyse the process in its natural environment,” Cramer says. “We want to watch the RNA polymerase at work.” It is still unclear whether this will be successful because new methods will have to be developed once again. To demonstrate what such future work will involve, Patrick Cramer shows an electron micrograph of portions of a cell nucleus. The DNA and its accompanying proteins appear as a granular mass – but there is no sign of the polymerase! At least for now, because Cramer’s department is currently testing a new approach that also involves cryo-electron tomography. In such tomography, researchers image the granular mass from different angles. “It will become difficult to see RNA polymerase directly,” Cramer explains. “Which is why we want to fit known structures, as if they were pieces of a jigsaw puzzle, into the lower-resolution tomographic reconstructions. With the help of artificial intelligence, not only are the images now getting sharper, but we can also predict the structure of the puzzle pieces. In light of this, we hope soon to see what a gene looks like during transcription.”
Making progress in the research is one thing, but Cramer is also trying to bring new knowledge to a wider audience. He writes essays for the general reader, gives public lectures, and sends out tweets. “It’s important to me that we explain what we do and why we need basic research.”

The urgent need for this kind of research was starkly demonstrated in the past two and a half years of the coronavirus pandemic. Since the outbreak of Covid-19, scientists have been at the centre of public attention like never before. But how to act in such troubled times, when fake news and conspiracy theories threaten public debate and democracy? Patrick Cramer sees science as an advocate for reason and the starting point for fact-based politics. “Especially on issues that are directly relevant to people, we need to take a stand. We need to state clearly what we know, but also what we do not know.”
Blocked polymerase should stop virus
His everyday life as a researcher has likewise been changed by the virus, since SARS-CoV-2 also contains an RNA polymerase. This polymerase is a great target for developing antiviral drugs because blocking it will stop the virus from replicating. Cramer remembers all too well the beginning of the pandemic in the spring of 2020: “We knew we weren’t able to help develop a vaccine, but we knew about polymerases and thus knew we might be able to help develop antiviral drugs – so we got to work.” Half a dozen employees immediately returned to the lab from their home offices – under the applicable safety regulations, of course. And thus began a race against time – and against research groups in China. The latter had gotten a jump start, but Cramer’s team was able to catch up. Moments of euphoria alternated with setbacks, but already in early April 2020 – just six weeks after the coronavirus reached Europe – the big moment had come: almost simultaneously, the teams from Germany and China published the structure of the viral polymerase. Unlike the data from Asia, the Göttingen results also showed the novel molecular hooks of the corona polymerase, which allow the polymerase to cling to the RNA template until it has copied it. This is particularly important for the coronavirus because its genome consists of around 30,000 building blocks and is thus particularly long for a virus, making copying a truly mammoth task.
In the months that followed, Cramer’s team also studied the effect of antiviral drugs. The researchers were able to show why the antiviral agent remdesivir, which was the first Covid-19 drug to be approved, has a fairly weak effect on patients. “Remdesivir does interfere with the polymerase in its work, but it does so only after some delay. And the drug does not stop the enzyme completely either,” explains Patrick Cramer.
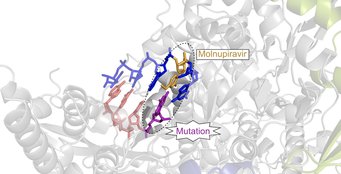
In contrast, molnupiravir – a compound originally developed as an influenza drug – acts quite differently against SARS-CoV-2. As the Göttingen researchers showed in detail, molnupiravir, in contrast to remdesivir, does not directly impair the function of the copying machine. Instead, the active ingredient ensures that mutations occur during replication of the viral genetic material, which means that the virus can no longer duplicate. Since the beginning of 2022, the drug has been in use in various countries to treat Covid-19.

In the third year after the outbreak of the pandemic, the excitement is picking up again in Göttingen: Cramer’s team is hoping to find an active agent that aggressively blocks the polymerase of the virus and thus inhibits the multiplication of the pathogen more effectively. For this, the researchers are collaborating with colleagues at the Max Planck Institute of Molecular Physiology in Dortmund. Together they have screened more than 300,000 substances for potential active ingredients that inhibit viral polymerase Yet the researchers are just starting out here: the Lead Discovery Center, also located in Dortmund, will now help bridge the gap between inhibitory substances and drug candidates, which is notorious among pharmaceutical researchers, and thus start the movement toward the development of new drugs. “The core of the Max Planck Society brand is basic research. At the same time though, we should ensure that new knowledge from which people can potentially benefit is also exploited,” Cramer emphasizes.
The promotion of innovative research will occupy Cramer even more intensively in the coming years – albeit less in his capacity as Institute Director, but rather as President of the Max Planck Society. In June, the Senate of the research organization elected him to be Martin Stratmann’s successor, and he is set to take up this office in June 2023.
What principles will he follow during his tenure? “For one thing, excellence means more than outstanding research results. Our standards must be higher, because not everything that can be measured counts. And not everything that counts can be measured. Excellence requires breaking new ground in research, daring to be bold. We have the privilege of determining where we go.” Equally important to the President-elect is nurturing the work culture: “As the Max Planck Society, we are only as good as the way we treat our employees. Promoting young talent and diversity is of crucial importance.”
Consistency with our values
Dealing with non-democratic states will be another challenge for the future President. It is a balancing act, as Cramer knows from his own experience. “We should find ways to jointly advance research that is important for people’s future, but we also need to clearly identify what is not consistent with our values. When doctoral researchers and postdocs come to us from abroad, they also learn something about our culture. That shapes young people and contributes to understanding.” And what about Russia? Cramer thinks it was good that the Alliance of Science Organizations suspended all collaborations with Russian institutes after the attack on Ukraine. Yet there’s not only the political level to consider here, but also the personal one: Cramer’s institute employs 27 Russian and ten Ukrainian staff. When the war began, Cramer wrote to the Russian employees to say they were still welcome, while to the Ukrainians he offered help. Many relatives of co-workers from Ukraine were subsequently given accommodation at Göttingen.
Research on transcription is now also being carried out by many of Cramer’s former employees in their own laboratories in various countries. This allows Patrick Cramer to prepare for the new post, as part of which he is currently traveling to all 85 Max Planck Institutes. “I want to know from people what their concerns are and what ideas and dreams they have.” The coronavirus, Russia’s war of aggression, rising costs for energy and construction—all of this also affects the Max Planck Society. “But that’s precisely why we have to develop positive future scenarios and new opportunities for action. If not us, then who?”
Summary
The enzyme RNA polymerase II is the central enzyme of transcription, in which a gene located on the DNA is transcribed into an RNA molecule. Deciphering the spatial structure of polymerase and the mechanism of gene transcription is a milestone in molecular biology.