2021 Animal Welfare Research Prize for Max Planck researchers
Max Planck researchers lay the foundation for the automated production and analysis of human 3D tissues for drug and toxicity research
Without animal experiments, it is nearly impossible to gain new insight into the brain’s inner workings. The development of new pharmaceuticals necessitates testing in laboratory animals. Jan Bruder and Henrik Renner at the Max Planck Institute for Molecular Biomedicine in Münster developed a method which could reduce the number of required animal experiments. The researchers generated three-dimensional tissue cultures from precursors of human brain cells. Over the course of a few weeks in the lab, these develop into pinhead-sized organ-like tissues called organoids. The two scientists have also designed an automated manufacturing process that allows them to produce and analyse the organoids in a standardized manner and in high numbers. These organoids can provide the raw human material in basic research on neurological diseases. In addition, drug candidates that prove to be ineffective or toxic to these organoids do not require further testing in animal experiments. For this technology, the Federal Ministry of Food and Agriculture awarded them the 2021 Animal Welfare Research Prize.

Center: entire organoid (red: markers for young neurons).
Right: center of organoid (blue: cell nuclei; red: young neurons; green: marker for neural stem cells).
In order to elucidate the functioning of the brain and develop drugs against Alzheimer’s disease, Parkinson’s disease, and depression, researchers must study the brains of laboratory animals. Nerve cells are traditionally grown in two-dimensional cell cultures derived from laboratory animals. These generally thrive in a nutrient solution and form a lawn of interconnected nerve cells on the bottom of their cell culture vessels. However, these two-dimensional cultures only roughly approximate the actual conditions in the human brain with its different cell types and three-dimensional networks. Moreover, these nerve cells originate from animals and physiologically resemble human brain cells to a limited extent.
The brain organoids from Münster are quite different. They arise from special neural precursor cells that spontaneously form pieces of human neural tissue and can form networks in all three spatial directions. These cells can produce the cell types relevant for the organoids particularly quickly and reliably. Because of their three-dimensional arrangement, they mimic the characteristic properties of natural brain tissue better than classical 2D cell cultures. In most labs, organoids are still largely produced individually by hand. As a result, they can vary greatly from organoid to organoid and batch to batch. They also cannot be produced in large quantities in this way. However, drug research needs large quantities of uniform organoids.
Focus on the midbrain

Jan Bruder and Henrik Renner have developed a method which allows them to generate organoids from different brain regions thousands of times in parallel in a manner suitable for drug screening and development. They focused on the midbrain – a region where many nerve cells die off in Parkinson’s disease for example. In a fully automated process, the scientists use a pipetting robot to create any number of organoids and allow them to develop to their desired cellular maturity. Thanks to the standardization of the workflow and the high uniformity of the samples produced, they can precisely analyse the effects of diseases and possible drug candidates or toxins on the resulting human nerve tissues.
Analyses have shown that the automatically produced midbrain organoids are at least as similar to the human midbrain as those produced by hand. “They contain many of the dopamine-producing nerve cells first damaged by Parkinson’s disease. These are electrically active and generate complex, spontaneously occurring and partly synchronous activity patterns”, explains Renner.
Model for Parkinson disease
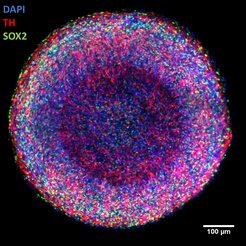
The high reproducibility of the organoids also allows researchers to study even the most subtle effects of diseases or toxins in organoids in an automated way. “We can use our midbrain organoids to observe the death of dopaminergic neurons in an active human brain-like tissue – the same cells that are preferentially damaged in Parkinson’s disease. They have great potential to become the next generation of a novel disease model for this condition. We can also determine the effect of toxins such as pesticides on the organoids in a fully automated manner”, says Bruder.
The researchers have designed their technology for use in the pharmaceutical industry. “I hope that in the medium term, our system will make at least some of the animal experiments in neurological and pharmacological research unnecessary. Active ingredients that have a toxic effect or which are not sufficiently effective in our experimental system could be excluded from development at an early stage and would no longer need to be tested in animals”, says Bruder.














