LInDA – disconnecting cells with light
An optochemical tool for precise control of cell-cell adhesions and study of collective cell behavior
Cells connect with each other, among other mechanisms, through adherens junctions (AJs). These play a major role in collective cell behavior and are relevant for many biological processes such as cell migration in wound healing.
The protein E-cadherin is located at the cell membrane of each cell. It spans the cell membrane, with an outer domain that connects to a counterpart on another cell and an intracellular part. The latter connects to the ‘skeleton’ of the cell. Upon binding to the ‘skeleton’, AJs between cells are formed. “We can now exchange some of the components of this complex, in order to manipulate the binding process,” says Ada Cavalcanti-Adam, corresponding author and departmental group leader at the Max Planck Institute for Medical Research. “We use a chemical compound to establish the link between adjacent cells and use light to break this link.” The researchers named the tool LInDA -”Light-Induced Dissociation of Adherens Junctions”.
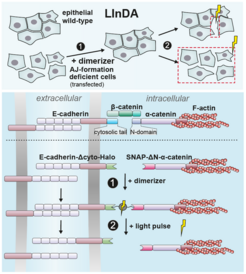
“LInDA allows us to control the formation and dissociation of adherens junctions with unprecedented spatial and temporal control,” says Dirk Ollech, first author of the paper and former PhD student in the Department of Cellular Biophysics at the Max Planck Institute for Medical Research. “It is similar to a switch that can be flipped on demand.” Upon adding a chemical dimerizer, AJs are formed – the switch is turned ON. The scientists are now able to apply a short light pulse at a precisely defined position to break the chemical bond – the switch is turned OFF. As a result, the AJ dissociates. The location of this AJ can be chosen with high precision, allowing detailed studies of the effects on a whole cell collective. This was shown in cell culture as well as in vivo. “The LInDA approach offers new possibilities to study the role of E-cadherins in cellular adhesion and cell migration, as well as cell-cell junction dynamics in embryonic development”, says co-corresponding author Richard Wombacher from the Heidelberg University.