How to herd atoms
Self-organization of atoms in circular atomic pens observed
It has long been known that it is possible to confine electrons or atoms in atomic structures in the same way as sheep can be shut in a pen. Physicists at the Max Planck Institute for Microstructure Physics in Halle have now discovered a strange thing: if the atomic fences have the right shape and the substrate, temperature and other parameters are adjusted appropriately, then randomly vapour-deposited atoms arrange themselves in regular structures within the circular fencing - as if they were sheep arranging themselves neatly in a pen (Physical Review Letters, 2nd November 2006).
For some years, numerous groups of researchers all over the world have been concentrating on forcing conduction electrons (the electrons used for the conduction of electronic current) on the surface of certain materials into patterns using deliberately planted atoms. Their intention is to influence the growth of thin films of material. When new atoms, called adatoms, are vapour-deposited on these electron structures, electrical attraction and repulsion makes them more likely to settle in some areas rather than others, depending on the density of electrons on the material. Physicists hope that they will be able to create thin films of material with predetermined characteristics by tailoring the density of electrons.
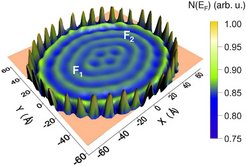
The researchers at the Max Planck Institute for Microstructure Physics together with physicists from the University of Halle and the University of Santiago de Compostella in Spain have investigated a special form of electronic structure. They observed electrons in a dense, closed ellipsis of cobalt atoms on a copper substrate. The conduction electrons can be imagined like a gas or a liquid; they form standing waves in circular atomic "pens" similar to waves in a small pond.
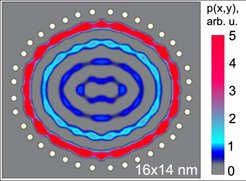
The physicists then simulated the effects of vapour-depositing cobalt adatoms. The new atoms interact with the cobalt atoms in the pen and with the enclosed electrons. There are tiny fluctuations in the energy levels which only have an effect at low temperatures of around 10 to 20 kelvins. These fluctuations cause the adatoms to prefer to move to positions with higher densities of electrons, provided the number of vapour-deposited adatoms is correct, the temperature is low enough and the pen sufficiently secure.

The cobalt atoms arrange themselves, so to speak, like the waves in a pond of electrons in ellipses. With adatoms, which can move more easily at lower temperatures, - for example atoms of the element cerium and a circular enclosure, the researchers created regular structures on the circles themselves; this was similar to allowing sheep to run randomly into a pen where they obediently line up, spaced at regular intervals and in concentric circles.
The next step will be to offer experimental proof of the simulations, which should be possible with current atomic scanning force microscopy, and to find new ways to create thin films.














