Shimmering Colours Which Change With Temperature
Max Planck researchers in Potsdam, Germany expand the tool kit of colloid particles and make new coloured finishes possible
Scientists at the Max Planck Institute of Colloids and Interfaces have used ion bombardment and gold metallisation to produce a new family of particles whose bonding behaviour can be chemically tailored. With these particles, scientists hope not only to be able to perform better research on the dynamics of solids and molecules. The discovery could also bring about, among other things, the development of new finishes which change their colour with temperature. (Angewandte Chemie, December2, 47/2005.)
Nail polish and expensive cars can nowadays shimmer in many colours, thanks to progress in the field of colloid chemistry, the chemistry of small particles. The bright colours in modern finishes are created because the light is reflected at layers of regularly arranged colloid particles. Individual colours are either removed or strengthened; the thickness of the layers -- what is known as the "lattice constant" -- determines the colour. Because we can nowadays tailor the spherical shape and the surface of the particles, we can produce optimised crystals with the desired lattice constant in the range of visible light.
Colloids can indeed do much more: they are also interesting model systems for solid-state physics, because the bonding behaviour of the relatively large particle can be compared with that of much smaller atoms. Since they react more slowly than atoms, we can use them to observe and study processes in solid-state physics. But there is a problem: most atoms, unlike most other particles, are not by rule spherically symmetric, but rather have deformed "orbitals" which project into space like dumbbells or ovals.
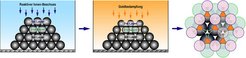
The team of researchers from the Max Planck Institute of Colloids and Interfaces, led by Dr Wang, has now produced particles that do not interact with their neighbours in spherically symmetric ways. So they placed a colloidal crystal on a surface (image 2) and bombarded it with reactive ions, reducing the particles in the upper layer to the desired size and expanding the free surfaces between the colloids.

They also metallised the crystal with gold. Part of the gold passed through the gaps in the upper layer as if through a stencil, all the way to the lower layers. In this way, patterns of metallisation of various symmetries and at nanoscale sizes are produced (see image 1). Gold surprisingly also lodged itself in the deep layers on the underside of the particles. (image 1, right)
For years, chemistry has had a number of methods to intentionally use gold in reactions, for example, in joining particular molecules. Thus the particles partially overlaid with gold expand the tool kit of "colloid atoms". The chemists hope that in the future they will be able to build "colloid molecules" or new kinds of colloid crystals. For the chemistry of colours, too, there are more possibilities: new, shimmering colours, that, for example, change with the surrounding temperature or humidity. In the long-term, however, the most attractive applications appear to be in optical data processing.

