Socioeconomic inequality in children’s epigenetics
Children growing up in under-resourced families have epigenetic profiles associated with worse health
To study the effects of childhood environments on adult health and well-being, researchers examined epigenetic profiles of children using algorithms developed in adults. They find that children growing up in more socioeconomically disadvantaged environments exhibited epigenetic profiles that, in previous studies of adults, were associated with worse health.
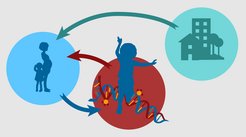
Children who grow up in socially disadvantaged families tend to experience more adverse environments, such as limited access to high-quality nutrition, green space, and health care. Accordingly, these children tend to be at increased risk for high body mass index (BMI), obesity, and multiple diseases in adulthood. The “long arm of childhood” is a phenomenon that describes the lasting effects of childhood environments and development on adult health and well-being.
“The idea is that what happens in childhood matters – to some extent – for life”, says first author Laurel Raffington, who conducted the study with collaborators at the University of Texas at Austin, the University of Michigan, and Princeton University. Raffington leads the Max Planck Research Group Biosocial – Biology, Social Disparities, and Development. “But understanding and intervening on the long arm of childhood is difficult because the effects of childhood environments can take decades to become visible in adult health and well-being.”
Raffington and colleagues used algorithms to summarize gene modifications across the entire genome into epigenetic profile scores. Epigenetic profiles, which turn genes “on” or “off”, can change as children develop and can be negatively affected by adverse environments. This epigenetic profile score was originally developed in adults to predict their body mass index (BMI); hence it is called epigenetic-BMI. Importantly, epigenetic-BMI was found to prospectively predict adult health and mortality beyond adults’ actual BMI. In the present study, researchers used that same algorithm to compute epigenetic-BMI in children, including over 3200 8-18-year-olds from two US studies. Unlike studies in adults that typically collect blood, they computed epigenetic-BMI using children’s saliva, which is less invasive and easier to collect. Because epigenetic modifications differ by tissue type, it was unclear whether epigenetic-BMI would be a valid measure when computed in saliva rather than blood DNA.
Epigenetic-BMI is associated with actual BMI
The researchers found that children’s epigenetic-BMI was strongly associated with their actual BMI. For example, epigenetic-BMI measured when children were 9 years old predicted their BMI when they were measured again at 15 years. Epigenetic-BMI was even related to differences in BMI between identical twins. “Longitudinal and twin analyses are very stringent tests of biomarker sensitivity, and we were impressed by how well epigenetic-BMI performs. Epigenetic-BMI is a valid measure of BMI even when it is measured in children’s saliva”, says Daniel A. Notterman, Professor of Molecular Biology at Princeton University.
They also found that children from more disadvantaged social backgrounds had higher epigenetic-BMI, even when controlling for their actual BMI, pubertal development, and tobacco exposure. Because the epigenetic profiles were computed using algorithms developed in adults, this indicates a molecular link between childhood environments and adult health. Socioeconomic status at birth was most strongly associated with epigenetic-BMI, which was pretty stable across adolescence. This may suggest that very early life environmental differences, such as those related to social inequality as well as those related to (prenatal) differences between identical twins, may have a critical influence on lifetime epigenetic profiles of later life health.
Using a similar procedure, recently published studies by Raffington and colleagues found that children growing up in under-resourced families tend to have epigenetic profiles associated with a faster pace of biological aging and lower cognitive health (see Raffington et. al, Psychological Science, 2023). Another study by Raffington and colleagues (see Raffington et. al, Clinical Epigenetics, 2023) suggests that these results are also applicable to Germany, though the full extent to which they generalize to other countries and contexts is not yet known.
Bridge between childhood and adulthood
The current study builds on previous work showing that epigenetic measures may be a molecular “bridge” between childhood and adulthood. It may be valuable for future studies assessing the impact of social policies in childhood to incorporate epigenetic measures as outcomes that are potentially informative about future health payoffs. Raffington is collaborating with the Babies First Years Study, a randomized controlled trial that has been giving cash gifts to mothers for the first four years of their baby’s life. They will observe whether receiving those cash gifts in early childhood improves children’s epigenetic profiles of health and well-being.
Read an accompanying interview with Laurel Raffington. She contextualizes the study results and speaks about future research projects. She also speculates about what her research implies for the current debate on basic child financial security (“Kindergrundsicherung”) in Germany.