Atomistic description for the role of Magnesium in biology
An improved atomistic model for computational studies of Mg2+ is mandatory for an in depth understanding of its interactions with biomolecules
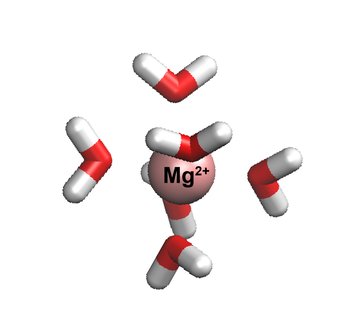
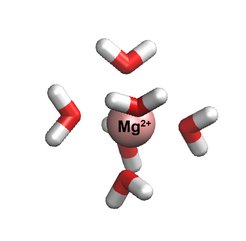
Magnesium plays an essential role in many vital processes. To correctly describe its mode of action in biological systems by computer simulations, accurate models with atomistic resolution are crucial. Researchers in the group of Nadine Schwierz at the Max Planck Institute of Biophysics developed an improved model for Magnesium for molecular dynamic simulations. The new model provides an unprecedentedly accurate description of behavior since it closely matches a broad range of experimental properties including the interactions with biomolecules. Moreover, it is shown that the model enables the discovery of important Magnesium ion binding sites on large biomolecules. The model therefore provides the first step to resolve the role of Magnesium in biological processes at the molecular level.
Magnesium is an essential nutrient and plays a vital role in many physiological processes. For example, it is needed in more than 300 biochemical reactions in our body and is essential for a healthy immune system or normal nerve and muscle functions.
However, resolving the role of Magnesium in these vital processes is challenging experimentally. Here, computer simulations can help a lot by providing unique atomistic insights. Motivated by the distinguished role of Magnesium, researchers around the world tried to develop accurate models for computer simulations at least over the last two decades. Yet despite the tremendous scientific effort, the models still showed significant shortcoming and an accurate description of Magnesium in biological systems remained out of reach.
Now Nadine Schwierz and her group developed a new model which provides a highly accurate description and closely matches a broad range of experimental properties including the interactions with biomolecules. “The shortcomings of classical force fields for metal cations have been a well-known problem in the simulation community”, Schwierz said. “We are convinced that our improved force fields provide an important step forward toward more accurate biomolecular simulations." The key to the successful parametrization of the model was to take a much larger parameter space into account and to include many body and quantum mechanical effects implicitly.
“The improved description allows us to reduce the computational time to simulate Magnesium binding from several hundred years to days or even hours” Grotz added. “This allows us to discover how Magnesium interacts with biomolecules.” Schwierz and her group could validate the de novo discovery of metal binding sites at an RNA-based riboswitch. The highly efficient and accurate model developed by Schwierz and her group therefore opens up the possibility to gain a detailed understanding of Magnesium ions in biological systems.