Water exchange in magnesium’s hydration shells impacts biological processes
Atomistic insights into the exchange dynamics on the millisecond timescale from transition path sampling
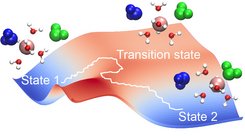
Free energy landscape of water exchange and simulation snapshots along the pathway of water exchange showing the two exchanging water molecules (green and blue) and the spectator water molecules of the first hydration shell.
In aqueous solutions, magnesium ions are surrounded by a hydration shell of six water molecules, which is subsequently enveloped by a second hydration shell. Water exchange between these shells plays an important role in biochemical processes. However, as the exchange dynamics is out of reach for conventional all-atom simulations, it remains poorly understood. Using path sampling, Nadine Schwierz investigated the water exchange dynamics between the two shells.
Schwierz found that the majority of these exchanges occur via an indirect mechanism. When a water molecule leaves the first hydration shell, another molecule from the second shell immediately fills its void, and the remaining molecules in the first shell collectively rearrange. Though the shells themselves do not have a functional role, the transfer of water molecules between shells impacts a wide range of biomolecular interactions.
“Water exchange governs every process in aqueous solutions that involves the replacement of strongly bound hydrogen water from the first hydration shell,” Schwierz said. “The mechanism is therefore essential for a large variety of biochemical processes ranging from simple ion pair formation to catalyzed reactions in metalloenzymes or the transport of ions across cell membranes.”
To characterize the mechanism of water exchange, Schwierz modeled a single Mg2+ ion surrounded by water molecules and used transition path sampling to collect and analyze a large number of exchange trajectories. This method provides insight on exchange dynamics and rates.
Though transition state theory overestimates the rate of the process, this can be addressed in the future by introducing additional degrees of freedom into the model. Schwierz noted this is a step toward improved atomistic Mg2+ models for biomolecular simulations, and the methodology is adaptable to other rare events.












