Biomineralization of molybdenum
Molybdate pumping into the molybdenum storage protein via an ATP-powered piercing mechanism
Basing on X-ray crystallography, cryoelectron microscopy, hydrogen-deuterium exchange mass spectrometry, and mutational studies the team of both Ulrich Ermler (Department of Membrane Biology) and Janet Vonck (Department of Structural Biology) showed that large amounts of molybdate can be stored in a protein so-called molybdenum storage protein (MoSto) in Azotobacter vinelandii, a gram-negative nitrogen-fixing bacterium.
"Our data support the occurrence of molybdate kinase and pyrophosphatase reactions in MoSto to pump molybdate into the locked inner protein cage against a molybdate gradient", so explained by Ulrich Ermler. The high molybdate concentration in the cage thus generated causes a protein-assisted self-assembly process of molybdate to polyoxomolybdate clusters by which approximately 130 Mo are deposited in a compact manner. "We believe that this molybdate pumping expands the known mechanistic repertoire of adenosine triphosphate, ATP-powered processes, since the chemical energy of hydrolysis of the phosphoric-molybdic anhydride formed would be conveyed onto the molybdate for penetration of the cage wall and not onto the protein for pore opening via conformational changes."
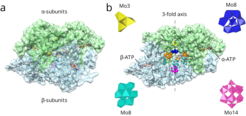
The MoSto of Azotobacter vinelandii is a heterohexameric (αβ)3 cage-like structure. The 3 α-subunits, related by a 3-fold axis, form one-half of the cage and the architecturally similar β-subunits form the other half in an equivalent manner.
Structure-function relationship
The cryo-EM map clearly reflects a MoStofunct state. Density is visible for the Mo3 cluster, covalent and noncovalent Mo8 clusters, 2 bipyramidal Mo8 aggregates, and a disordered Mo5 cluster. ATP and presumably Mg2+ bind to the α-ATP– binding site in a virtually identical fashion as found in the X-ray structure. The β-ATP–binding site also contains ATP but encloses a density beyond the γ-phosphate moiety. In addition, the β-ATP together with the expanded segment β193 to β225, enveloping the adenosine moiety, is shifted toward the cage wall by approximately 1.5 Å compared with the P6322 X-ray structure.
Most interestingly, the N-terminal arm (residues 3 to 36) of subunit α, disordered in the P6322 X-ray structure, is well ordered in the EM structure (at 3.2-Å resolution). The αN-terminal arm is arranged in a thread-like conformation (with 1 small helical segment) that wraps around the β-subunit and thereby shields the triphosphate of β-ATP from the only solvent-accessible side. The terminal residues α6 to α11 interact with subunit β and with subunits α and β of the adjacent dimer. This study on the cage-like molybdenum storage protein (MoSto) provides detailed insight into how nature realizes molybdenum biomineralization.