A vocabulary of ancient peptides
Max Planck scientists identify fragments of proteins that already existed billions of years ago
Proteins and languages share many similarities – both, for instance, yield their meaning through a proper arrangement of basic building blocks. Andrei Lupas, Director at the Max Planck Institute for Developmental Biology in Germany, and his team apply computational methods to reconstruct primordial building blocks by comparative studies of modern proteins. The same approach is used in linguistics to reconstruct ancient vocabularies through the comparison of modern languages. In a recent study the scientists report the identification of 40 ancestral, peptidic fragments, which possibly represent the observable remnants of a time when the first proteins were created, more than 3.5 billion years ago.
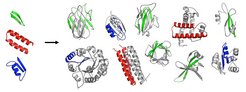
Proteins are integral building blocks of all life, from bacteria to humans. In our bodies, they are essential for all chemical processes: they form our nails, hair, bones, and muscles, they help digest the food we eat, and they defend us form pathogenic bacteria and viruses. “Life can be viewed as substantially resulting from the chemical activity of proteins”, says Lupas, Director of the Department of Protein Evolution at the Max Planck Institute for Developmental Biology. He and his collaborators are particularly interested in understanding how these complex biomolecules originated. Today we know that proteins are primarily built through the combinatorial assembly of only a few thousand modular units, termed domains. It is however unclear how these modular units themselves emerged.
The scientists investigated the hypothesis that the first protein domains arose by fusion and piecemeal growth from an ancestral set of simple peptides, which themselves emerged in an RNA-based pre-cellular life, around 3.5 billion years ago.
In a systematic analysis of modern proteins, they were able to identify 40 peptidic fragments that occur in seemingly unrelated proteins, yet bear striking resemblance in their sequences and structures. Based on their widespread occurrence in the most ancient proteins (e.g., ribosomal proteins) and on their involvement in basal functions (e.g., RNA-binding, DNA-binding), the authors propose that these fragments are the observable remnants of a primordial RNA-peptide world, a precursor form of the DNA-based life we know today.
In the future, the contribution of these fragments to the formation of protein structure will have to be investigated experimentally, opening new avenues to optimize existing proteins and design new ones, not yet seen in nature. "If we elucidate this process, we should be able to create new protein forms”, concludes Lupas, with exciting applications to biotechnology.
TS/HR
