How smart, ultrathin nanosheets go fishing for proteins
Faster and easier production of high-resolution, three-dimensional electron microscopy images of biomolecules
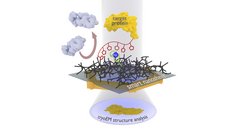
The new nanosheet process: The protein complex to be examined (yellow) is attached to the smart nanosheet via a nickel complex with the aid of a marker (red chain with pentagons). Undesired proteins (grey) are rejected by the hydrogel (black grid). After freezing the entire structure, including a thin film of water, this can be irradiated with electrons to obtain images of the bound proteins. From these images, a computer can calculate the 3D structure of the protein.
“With our process, new types of proteins can be isolated from mixtures and characterized within a week,” explains Daniel Rhinow from the Max Planck Institute of Biophysics. “To date, just the isolation of the proteins was often part of a doctorate lasting several years.” Together with Andreas Terfort (Goethe University Frankfurt) and Andrey Turchanin (Friedrich Schiller University Jena), the idea evolved a few years ago of fishing the desired proteins directly out of mixtures by equipping a nanosheet with recognition sites onto which the target protein bonds. The researchers have now succeeded in making proteins directly available for examination using electron cryo-microscopy through a “smart nanosheet”.
Cryo electron microscopy is based on the shock- reezing of a sample at temperatures below minus 150 degrees Celsius. During this process, the protein maintains its structure, no interfering fixing and coloring agents are needed, and the electrons can easily irradiate the frozen object. The result is high-resolution three-dimensional images of the tiniest structures – for example of viruses and deoxyribonucleic acid (DNA), almost down to the dimension of a hydrogen atom.
For preparation, the proteins are shock frozen in an extremely thin layer of water on a tiny metal grid. Previously, the samples had to be cleaned in a time-consuming and often costly process prior to their examination by electron microscopy. The electron microscopy procedure is only successful if just one type of protein is bound in the water layer.
Turchanin's group now uses nanosheets that are merely one nanometre thick and consist of a cross-linked molecular self-organising monolayer. This nanosheet provides Terfort’s group with a gel former as the basis for the thin film of water needed for freezing. The researchers then attach recognition sites (a special nitrilotriacetic acid compound with nickel ions) to this. The team led by Rhinow uses the “smart nanosheets” prepared in this way to fish proteins out of a mixture. These were marked beforehand with a histidine chain with which they bind to the recognition sites; all other interfering particles can be rinsed off. The nanosheet with the bound protein can then be examined directly with an electron microscope.
“Our smart nanosheets are particularly efficient because the hydrogel layer stabilizes the necessary thin film of water and at the same time suppresses the non-specific binding of interfering particles,” explains Julian Scherr from Goethe University. “As a result, molecular structural biology can now examine protein structures and functions much faster”. With the knowledge gained from this, diseases and their treatment with drugs, for example, can be better understood.
The team has patented the new nanosheets and has found a manufacturer who will bring this helpful tool to market.
