Fluorescent probes for imaging live cells
Scientists introduce new molecular labels for super-resolution microscopy
Researchers in the Department of Chemical Biology at the Max Planck Institute for Medical Research in Heidelberg have developed a new strategy for synthesizing cell-permeable and fluorescent probes for multi-color super-resolution microscopy in live cells. The so-called MaP (Max Planck) probes enable the fluorescence labeling of proteins in living cells with minimal background and excellent specificity, making them powerful tools to study biological phenomena on a molecular level.
Probes used in life-cell fluorescence imaging must be able to penetrate cells and bind specifically to the protein of interest. Ideally, such probes also increase their fluorescence intensity when binding to their target as this increases the signal to noise ratio. The latter property is called fluorogenicity, as the probe becomes (more) fluorescent when binding to its target. Kai Johnsson and his group specialize in the search for such molecules, and now introduce a generally applicable strategy that represents a major advance in the quest for fluorogenic probes for live-cell imaging.
“Our strategy was to enhance and tune the properties of existing fluorescent probes to increase fluorogenicity and cell permeability,” says Lu Wang, first author of the paper and a postdoc in the department of Kai Johnsson at the Max Planck Institute for Medical Research. Lu Wang initially focused on 6-TAMRA, a well-known rhodamine derivative. This fluorophore exists in a hydrophilic and fluorescent zwitterion ‘open’ form and a hydrophobic ‘closed’ form that is non-fluorescent but can penetrate cells. However, the equilibrium for regular 6-TAMRA greatly favors the fluorescent and zwitterionic open form and 6-TAMRA derivatives generally show low cell permeability.
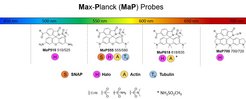
By introducing electron-deficient amides into 6-TAMRA, Wang was able to shift the equilibrium towards the closed form, to maximize permeability, while binding to the target shifts the equilibrium back to the fluorescent open form, resulting in fluorogenicity. These new probes, dubbed MaP (Max Planck) probes, showed vastly improved performance in live-cell imaging relative to the widely-used 6-TAMRA derivatives. Motivated by these results, Wang and his colleagues applied this simple strategy to improve the performance of various fluorescent probes that target different structures and proteins in living cells. The resulting MaP probes span the entire spectrum of the visible light and can, for example, be used for live-cell imaging of cytoskeletal proteins such as actin, tubulin and intermediate filaments as well as other cellular structures such as mitochondria or nuclei with confocal as well as super-resolution microscopy.
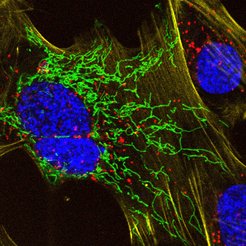
“A crucial benefit of the new MaP probes is that their high fluorogenicity allows their use without any washing steps and that we have an entire palette of colors available,” says Lu Wang. To highlight these features, the scientists in the Department of Chemical Biology conducted an experiment where live cells were incubated with four different probes and imaged directly without any washing steps, providing detailed insights into the dynamic behavior of proteins and cellular structures. “We are excited to provide the scientific community with a new tool to study important biological questions”, says Wang. While applying their new MaP probes for applications in live-cell imaging, he and his colleagues will also continue to use their expertise in synthetic chemistry to design other probes for visualizing the central molecules of life.