Connecting Science and Business
The figures are impressive: To date, 4,975 inventions have been managed, ca 3,000 commercialization agreements signed, 190 company spin-offs have successfully been supported and revenues totaling more than 585 million euros recorded on the books. Max Planck Innovation, the technology transfer arm of the Max Planck Society, successfully mediates between science and industry.
Text: Tim Schröder

The Max Planck Society conducts basic research. That is its task. At its institutes scattered throughout Germany, astronomers listen to the echo of the Big Bang, anthropologists try to understand the growth of the Homo erectus brain, and materials scientists, crack propagation velocity. The researchers get to the bottom of things. They want to explain the world, and sometimes they unearth findings that change our view of the world. “Free and independent” is how their work is to be. That is what the Statutes stipulate.
And indeed, some research projects appear to be so free, independent and, at the same time, so far removed that they seem almost ethereal, like the cosmic dust clouds in which new stars are born – which, incidentally, is likewise a topic for the Max Planck researchers. But that is just one side. After all, the Max Planck Society produces more than just concentrated knowledge. It also produces numerous patents and inventions with practical value, as well as ideas that drive business development and form the basis for new products that benefit many people.
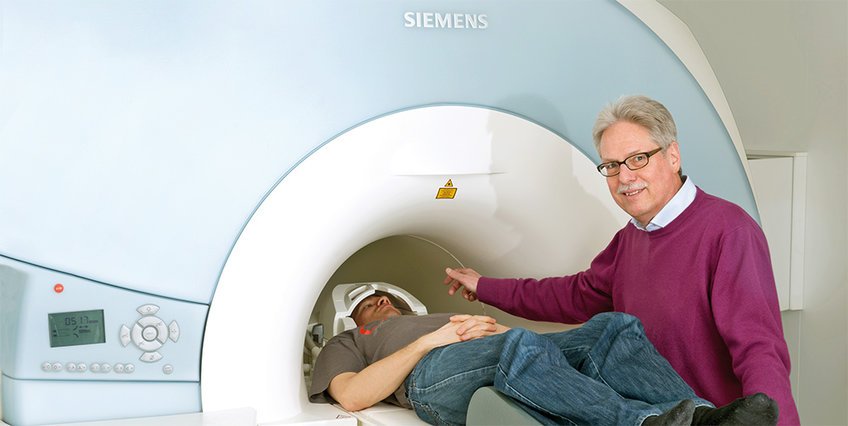
What is probably the most impressive invention of this kind has since reached a ripe old age: a patent application for Flash (Fast Low-Angle SHot) was filed in 1985. At the time, the publication and patent application struck manufacturers of magnetic resonance imaging scanners around the world like a thunderbolt. These devices, abbreviated MRI scanners, excite hydrogen atoms in the body and, based on their echoes, calculate images of the organs. In this way, diseases can be identified from outside the body, without an operation.
Back then, the usual instruments took more than an hour to image individual sections of a patient’s body. Thanks to a new measuring method, the Flash technique cut this time down to just a few minutes and was so fast that, for the first time, it was possible to capture moving images of the heart – a true sensation.
“This development was so dramatic that, from then on, no manufacturer could live without it,” says Jens Frahm, head of the former Flash team at the Max Planck Institute for Biophysical Chemistry in Göttingen, “and of course that was an exceptionally good market position for us.” But it would be years before the scientists would reap the rewards of their development. They first experienced their very own business whodunnit with Flash.
Dispute over Flash-patents
Rarely are basic researchers experienced business professionals. Not all of them are familiar with the tough rules of patent law. That is why, in 1985, Frahm and his colleagues relied on the support of Garching Innovation, as the Max Planck Society’s technology transfer company was known at the time. And a wise move it was. GI – which was renamed to Max Planck Innovation (MI) in late 2006 – mediates between two worlds, between business enterprises and basic researchers. In 1985, the innovation advisors had 15 years of experience in technology transfer issues under their belts and had already transferred many developments to companies in the form of licenses.

But the tide turned when the patents were finally granted in 1987 in the US and in 1989 in Europe. General Electric, Siemens and Philips filed oppositions to the patents, but continued to use the invention just as before. Agreements were ignored. Philips even went one step further and requested the invalidation of the granted patent in the US. For GI and Frahm, there was only one thing they could do: sue the companies for patent infringement.
“I don’t think the companies’ legal departments saw that coming,” says Bernhard Hertel, who was Managing Director of GI until the end of 2005, and the driving force behind the defense of the Flash patents. The companies pulled out all the stops and dragged out the proceedings: sources were cited incorrectly, new apparent counterevidence submitted time and time again. The battle cost money – a lot of money that was also urgently needed for other GI projects. Through 1991, the negotiations burned through 350,000 euros. “In addition, we constantly had to be on guard and make sure that our attorneys actually understood the complex technology in order to be able to argue cogently,” says Frahm.
But GI and Frahm stood firm – despite being outnumbered. Everyone knew that there was a lot at stake. Just how much became clear when the courts finally handed down their judgment in 1993: the companies had to pay licensing fees – retroactively. Up to that point, the Max Planck Society had invested nearly 1.5 million euros in the legal proceedings. It paid off, because the judgment has brought the Max Planck Society revenues totaling around 155 million euros to date – by far the largest sum in the history of GI or Max Planck Innovation. The patent has since expired. However, the technology still forms the basis of all new magnetic resonance imaging scanners today.
“Flash is currently the most significant technology transfer case in the history of the 39-year-old Max Planck Innovation, and not just in financial terms,” says Jörn Erselius, the new Managing Director and 15-year veteran of the Max Planck subsidiary. “In addition, the patent dispute brought us enormous international recognition.” For example, many industrial companies today view Max Planck Innovation as a competent and influential authority. This is important for future inventions from the labs of the Max Planck Society. Normally, an idea resulting from basic research requires investors who are willing to shoulder the risk to nurture it until it becomes a mature product. These investors must be courted.
But Erselius and his colleagues must also promote their work within their own company – to convince researchers of the importance of technology transfer. This is no easy task, “because many measure the value of their work in terms of publications in renowned magazines, not patents and licenses,” says Erselius.
But things are happening: “Until the 1990s, technology transfer in the Max Planck Society had, for the most part, led a shadowy existence. Now, however, many see it differently,” says the Managing Director. Examples such as Flash may have contributed to this. “When it comes down to it, the goal of noble, free basic research hasn’t changed – we are simply trying to make it clear that one can still do other things on the side.” Like commercializing inventions.
Max Planck Innovation supports scientists in translating research into practical application
Max Planck Innovation does this, in collaboration with the researchers, in two ways: through licensing agreements and spin-offs. The team has 24 members – biologists and physicists, lawyers, business experts, and a chemist. “Our scientists understand the language of the researchers, but they are also trained in matters of patent law and patent and license management,” says Erselius, who himself is a biologist who also has an MBA degree. And one more trait is crucial: they must be able to sell. After all, anyone who sits down with the heads of business development of major companies should be able to persuade them, and to present the invention in an appropriate manner.

The Max Planck Innovation team relieves scientists of a significant portion of the need to make external contacts: they negotiate commercialization agreements, handle licensing matters, defend patent rights and assist in drawing up business plans for spin-offs. Since the collapse of the new market in 2001, venture capital investors have become very cautious. Says Erselius: “Only in exceptional cases will single-product companies likely make it in today’s market. Usually they must make it clear that the new technology has broad applications and that it offers promise for other new products, in case one turns out to be a failure.” The business plan must take this into account. But even if this condition is met, the latest financial crisis – after a slight temporary recovery – has brought further cuts, such that even the existence of young, established companies, for example in the biotech industry, is threatened.
This makes it all the more important to provide support in setting up companies, which is why Max Planck Innovation also helps with putting together the right team for the new business. After all, a young company can persuade investors only when the top-rate researchers are accompanied by a capable management team to run the business. “There simply must be expertise on both sides. We try to bring the two together,” says Jörn Erselius.
Sunitinib - an example of successful knowledge transfer
Concentrating on research and leaving business matters to reliable partners – that is how Axel Ullrich has always done it, too. “My calling is definitely not to be an entrepreneur. I prefer to leave that to people who are good at it, and who also understand my work,” says the Director at the Max Planck Institute of Biochemistry in Martinsried, near Munich. Nevertheless, the biochemist and developer of cancer drugs is probably one of the most enterprising scientists in the Max Planck Society. In about 30 years of research work, he has established 4 companies and filed applications for 60 patents.

One of Ullrich’s most successful developments is the breast cancer drug Herceptin®, which stems from his time with the American biotech company Genentech, which was founded in the late 1970s. In the meantime, Sutent®, a new cancer drug, has achieved a breakthrough. It may even become a blockbuster this year – that is what pharmaceutical companies call drugs that generate more than a billion dollars in annual sales. Sutent® could thus become a success the likes of Flash – and, like the latter, it is a further example of how many years can pass from the time an idea is developed until it becomes a successful product.
The genesis of a drug takes time and culminates in expensive clinical trials that hardly any research institution can shoulder alone. Sutent®, too, would not have existed without the influence of a major pharmaceutical company. Sutent® (or, more accurately, the active ingredient Sunitinib) is a multikinase inhibitor that simultaneously flips multiple cellular switches that are important for the growth of tumors and of the blood vessels that supply the tumors. The active ingredient blocks receptors on the surface of cancer cells. If certain molecules – so-called growth factors – dock at these receptors, the receptors trigger a series of fatal signals. Sunitinib prevents this, and the tumor dies.
For Axel Ullrich, this cellular metabolism mechanism had become clear as far back as the early 1990s. It was just as clear that he would need support if it was to ever become a drug. “It was very important to me that I develop the idea into an application myself,” says the Max Planck researcher. So licensing it out wasn’t an option. Ullrich decided to set up a company with his colleague Joseph Schlessinger. In Germany, hardly any companies were interested in biotechnology research at that time. The two thus decided to base their company in the US. New York University signed on as a cooperation partner.
Ullrich wanted to avoid going it alone and inquired at GI. The then Managing Director Heinrich Kuhn believed in Ullrich’s idea, backed the company and finally managed to get the Max Planck Society to co-found the new company Sugen. Sugen was later sold to the Swedish company Pharmacia, which was subsequently taken over by Pfizer. The pharmaceuticals giant drove the clinical trials forward in record time. However, Max Planck Innovation made sure that the Max Planck Society and Ullrich retained their shares in the Sutent® business. Pfizer, too, remains obligated to pay sales-based licensing fees. For the next few years, Sutent® is likely to bring the Max Planck Society licensing revenues in the millions.
Sutent® clearly points up how important it is to safeguard valuable knowledge to avoid coming up empty-handed later. That is why Jörn Erselius constantly admonishes researchers to apply for patents to protect the findings they obtain through long years of arduous work. He knows that many researchers nevertheless hesitate. “For many of them, patents and publication in scientific journals are contradictory terms – so the researchers shrink away from applying for patents.” They fear that the protracted procedure will delay publication, as publication is not permitted before the patent application is filed.
Gap between basic and applied research
But that is only partly true. “The publication ban,” Erselius explains, “applies only up until the day on which the patent application is submitted – after that, nothing else stands in the way of publication.” However, it is important to inform the patent attorney of the date of the planned publication. “Ultimately, what it comes down to is simply the right sequence of publication and patent application.”
However, the greatest obstacle on the path from development to product is not usually the patent, but rather the lack of funds. Only in the rarest of cases does basic research produce fully finished ideas. This is simply how it is. But investors want mature developments, ideally prototypes from which they can tell whether they will be a financial success.
“There is an innovation gap between the two sides,” says Jörn Erselius. “Germany’s federal and state governments spend some 20 billion euros in support of science each year, of which about half goes toward basic research. A fraction of this amount would be sufficient to develop many discoveries and inventions to the point that they could be picked up by industry. But there has, as yet, been too little funding, and the findings of basic research are not being turned into innovations to the extent that would be possible.
This is a great loss, because it is precisely these findings that form the basis for new top-ranking products. In the field of biotechnology, the Life Science Inkubator at the caesar research center, and the MI-founded Lead Discovery Center in Dortmund are now helping to close the innovation gap. For inventions that go beyond biotechnology, the Federal Ministry of Education and Research is considering a validation fund that would, as a central institution, provide resources for scientists from research facilities, universities and polytechnic institutes. However, unlike traditional applications – for example to the German Research Foundation – here, not only the scientific basis would be assessed, but also the future market and competition. Approval needs to be quick and unbureaucratic.
In this way, the fund would satisfy a condition that researchers like Jens Frahm have long been calling for: “We need a fund that supports projects spontaneously and unbureaucratically across geographic regions and disciplines – especially unconventional projects, because it is often precisely the bizarre theories that yield revolutionary innovations.” This requires a leap of faith. Frahm criticizes the traditional funding policy, the tedious “over-evaluation” of applications. “Applications have since reached book format. Who can even process them anymore?”
There are now a few support programs available for young researchers who want to set up their own companies to market their findings, such as the Federal Ministry of Economics and Technology’s EXIST-Forschungstransfer, the Kreditanstalt für Wiederaufbau’s High-Tech Startup Fund and the Federal Ministry of Education and Research’s ExistGo-Bio program.
Some 20 years ago, there were hardly any such programs in place – nor was there much demand for them. Naturally, Max Planck Institutes already existed back then – such as the one for iron research in Düsseldorf – and conducted research that was directly relevant to industry. “On the whole, however, science was driven by hypotheses rather than by technology. Applied research was considered distasteful,” says Frahm. But the world has changed. Now, it is expected that the major research institutions that receive government funding give back more to society than just scientific results.
Max Planck Innovation - a success story
As for Max Planck Innovation, it has already given back a lot. Since 1970, it has accompanied 4,975 inventions on their path to market and concluded more than 3,000 commercialization agreements. To date, it has posted licensing revenues totaling more than 585 million euros – a large part owing to Flash alone. Since 1990, the company has advised start-up founders and has so far helped over 190 companies get off the ground. Based on its licensing revenues, says Erselius, it ranks right up there with the Fraunhofer-Gesellschaft as a leader among German technology transfer institutions. And Max Planck Innovation would be in the first league in the US, as well.

This is also likely to remain the case in the future, as new and promising companies are currently getting started in which Max Planck Innovation is involved. One example is Alnylam. One of the founders of the company is Thomas Tuschl, a former researcher at the Max Planck Institute for Biophysical Chemistry in Göttingen, now working at Rockefeller University in New York. Tuschl is one of the scientists who, in the late 1990s, discovered and established RNA interference – a completely new method for silencing genes that cause disease.
The scientists noticed that gene activity in a cell is determined in part by small RNA molecules that research had, until then, largely neglected. Tuschl analyzed the structure of these double-stranded RNA molecules (si-RNAs, small interfering RNAs) and, for the first time, succeeded in demonstrating their impact in mammalian cells. By using siRNAs, genes can be systematically silenced. The RNA interference (RNAi) method is now used in labs throughout the world to study the function of genes in cell cultures and lab animals. A therapeutic effect has already been proven in various clinical studies. It is quite possible that future RNAi drugs will be as promising as Sutent®. Axel Ullrich is hardly surprised that many biochemical findings are making their way into practical applications. “This field just happens to be very application oriented, it is hardly possible to avoid the opportunities for future therapies.”
But there are also examples of basic knowledge that have found their way into earthly applications from very distant places – like outer space, for example. Normally, colleagues of Gregor Morfill, emeritus Director at the Max Planck Institute for Extraterrestrial Physics, spend their time gazing into the depths of the cosmos. These Munich-based scientists use mathematical methods to describe the distribution of galaxy clusters and the arrangement of matter in the vast expanse of the black firmament.
A few years ago, the researchers met with medical specialists from the Technical University Munich and discussed the question of whether their mathematical model might not also be suitable for studying the human body: galaxies are not evenly distributed throughout space, but are organized into structures that resemble those of a sponge, which contains many hollow chambers separated by thin walls. Human bones also have a very similar structure.
Thus, the astronomers’ discoveries are also good for everyday applications. And other ideas, too, can look forward to going to market: Max Planck Innovation evaluates roughly 150 new inventions every year and files patent applications for about half of them. It markets another 60 or so without patent protection – a sign that basic research is now closer to our daily lives than ever before, even when it sometimes seems so far removed.
Contact
Amalienstr. 33
80799 Munich
Germany
Telefon: +49 89 29 09 19-0





