On track for therapeutic uses
Tests on mice have shown, that genome editing can be used to delete faulty gene sequences like those that arise in human hereditary diseases, for example Duchenne muscular dystrophy, tyrosinaemia and Leber’s congenital amaurosis. Many medical researchers therefore firmly believe that gene editing methods will enable them to treat hereditary diseases, in which one or more genes do not function correctly.
CRISPR-Cas9, Zinc finger nucleases, and TALENs have by now been tested on HIV and cancer patients in various clinical trials. Moreover, the fact that gene editing can also be successfully applied in humans is demonstrated by studies on patients with Duchenne muscular dystrophy. Following CRISPR-Cas9 treatment, these patients presented slightly elevated levels of the missing protein.
HIV patients could also benefit from the method. In one study, scientists used a zinc finger nuclease to remove the gene for an immune cell receptor that is indispensable for the infection so that the virus could no longer infect and destroy the patients’ cells. The patients had more of these immune cells in their blood for weeks after the procedure. In addition, researchers have already successfully removed part of the virus DNA from the DNA of human cells using CRISPR-Cas9. It would appear, however, that the pathogens quickly become resistant to the gene scissors.
Some researchers also consider genes in the germ line – egg and sperm cells – a promising target to edit faulty genes so that embryos from in vitro fertilization will not receive inheritable diseases. For ethical reasons, gene editing in the germ line is controversial, however. Many scientists refuse such interventions as they change the genome of future generations.
Advantages and difficulties of genome editing

Without genome editing, scientists were only able to treat patients with a defective gene by inserting a new functioning gene into the patients’ infected cells using viruses. The new gene was intended to rectify the faults in a protein, which could not be produced by a defective gene or could only be produced in insufficient quantities. However, with classical gene therapy, the researchers are unable to predict the exact location in the DNA where the viruses will insert the gene. Which is why the insertion process could activate an otherwise silenced cancer gene. For this reason, patients with weak immune systems who were treated using gene therapy had a higher risk of developing leukaemia.
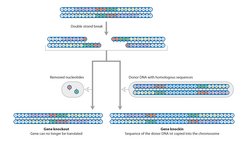
The editing of already existing genes could minimize this risk. As in classical gene therapy, viruses could be used to transport the DNA for the guide RNA and Cas9. This would also enable the treatment of diseases, in which a gene defect gives rise to a defective and thus pathogenic protein. In such cases, the insertion of new DNA is of no assistance – the gene for the protein that actually causes the disease must be removed. Examples of such conditions include certain immune diseases and sickle cell anaemia.
However, gene repair only works in comparatively few cells. The editing out of a defective gene sequence is not enough on its own. The cell must also restore the two ends of the DNA strand correctly and join them. This repair mechanism only works in dividing cells, but the majority of the body’s cells, like neurons, muscle cells, and liver cells do not divide.
Another difficulty arises from the fact that although CRISPR-Cas9 can locate its target with extreme accuracy, on rare occasions it can miss it. This could cause cuts in the DNA at undesired sites in the patient’s genome, the consequences of which are unpredictable. Moreover, the CRISPR-Cas9 could remain active in the cells for years after the administration of gene therapy. This increases the risk that the gene scissors could become active again at a later point in time. Even if tests on mice have shown that it has no apparent impacts even 20 generations later, it would be safer if CRISPR-Cas9 were only temporarily active.
Viruses as gene ferries
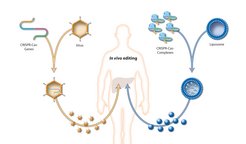
Various obstacles remain to be overcome before CRISPR-Cas9 can be used in human patients on a larger scale, however. One of these is the question as to how the gene scissors can be transported to its site of action. Could the gene scissors be injected or simply administered in the form of a pill? Neither of these possibilities are an option, as RNA molecules and enzymes like Cas9 are unable to penetrate the membrane of a human cell. Moreover, they would be destroyed in the digestive tract and attacked by immune cells in the bloodstream.
Medical researchers therefore need transport vehicles, with which they can deliver the CRISPR-Cas9 directly into the cell interior. One possibility would involve the use of nanoparticles, for example liposomes. These fat droplets, which are just one millionth of a millimetre in size, could be used to encapsulate the guide RNA and Cas9 enzyme. Once they are protected by the lipid membrane, both molecules could be transported through the bloodstream to the cells with the genetic defect. Receptor molecules in the membrane would ensure that the liposomes dock at the target cells and be able to pass their contents on in the cell interior. However, such a method remains pie in the sky at present, as nanoparticles have not yet reached a stage at which they would be suitable for medical use.
Unlike nanoparticles, which are still being developed, viruses are already being used as transport vehicles in gene therapy. After all, they are designed by nature to lock into their hosts’ cells, infiltrate their own genetic material and sometimes even build it into the cell genome. Doctors use naturally harmless or artificially inactivated viruses for gene therapy. In most cases, they remove cells from the patient and treat them in a cell culture with the viruses. Gene therapies for diseases like severe combined immunodeficiency (SCID), Leber congenital amaurosis and adrenoleukodystrophy have already been tested on patients.













