New stem cells for medicine
The CARE research institute in Munich is set to pursue new approaches in regenerative medicine and drug development
Growing organs from skin cells, replacing animal research with innovative toxicity tests and stimulating human tissue to heal itself – all of this could be possible thanks to stem cell research. Scientists at the “Center for Advanced Regenerative Engineering (CARE)” are seeking to develop technologies and treatments to use stem cells in medicine. The Bavarian state government is supporting the Center, which is scheduled to open in January 2017 in Munich, to the amount of 15 million euros. In April, the researchers presented the CARE institute to representatives from politics, business and science for the first time.
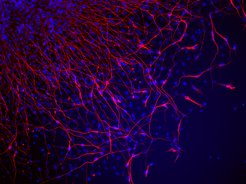
“Refine, reprogram, regenerate” is the mission of the institute established by Max Planck scientist Hans Schöler. Here, scientists will be optimizing the manufacture of induced pluripotent stem cells (iPS) and developing medical applications from them.
Human tissue and organs can be grown from iPS cells, which, thanks to their pluripotency, can be developed into many cell types. The tissue and organs could in turn have a variety of applications in medicine; they could be implanted in patients, for example, to replace diseased tissue or damaged organs. The findings that the researchers at the CARE institute draw from their work on iPS cells could also lead to the development of completely new therapeutic approaches in regenerative medicine: scientists want to use these to develop strategies to stimulate diseased tissue in the patient's body to heal itself. In this way, they hope to be able to treat metabolic, brain and heart disease. When, for example, heart muscle cells die away during a heart attack, or brain cells degenerate as a result of conditions such as Parkinson's disease, the body cannot regenerate new cells – the tissue remains damaged. With the help of iPS research, however, doctors could perhaps stimulate its regeneration.
Human tissue used to develop drugs
The CARE researchers would like to use the stem cells, however, primarily to generate cell types that were previously rarely available or not available at all. They can then use these in the laboratory to identify, test and improve candidates for pharmaceutical drugs. Experiments using human tissue can frequently predict the effects and side effects of substances more effectively than animal research, which could thus be reduced. “It would save a lot of time and money, and at the same time open up new avenues if new drugs could be discovered with greater accuracy and drugs with unwanted toxicological side effects be excluded at an earlier stage. The cells generated from iPS cells are ideal test candidates for this,” says Hans Schöler, who, as Director at the Max Planck Institute for Molecular Biomedicine in Münster, conducts research on stem cells and how they are obtained.

Scientists have already overcome two major hurdles to make stem cells a viable option for application in a medical context in the first place. Firstly, they can manufacture stem cells from somatic cells and therefore do not need to rely on the ethically controversial harvesting of embryonic stem cells. Secondly, the iPS procedure allows for the manufacture of large quantities of stem cells as they are needed for medical purposes. This is because normal skin, hair and blood cells, which are available in large quantities and are easy to harvest, can be used as the source material for manufacturing iPS cells. Using specific genes or biological messengers, the scientists reprogram these as stem cells in the test tube. In precisely defined culture conditions, the induced pluripotent stem cells can grow into a variety of cell and tissue types – a step that demands special skills. Although the method has been constantly improving since 2006, it has not yet been used in a medical context. This is about to change thanks to the CARE institute.
Cooperation with the Lead Discovery Center in Dortmund
In order to launch new regenerative treatments for use on patients and in drug tests, the research institute will work with partners such as the Dortmund-based Lead Discovery Center (LDC). The translational research center, which was spun off in 2008 from the technology transfer company Max Planck Innovation, already has several years of experience in drug research and development. Just like the CARE institute, it forms a bridge between basic research and medical application. To date, mainly small molecules have been tested for potential applications in medicine. As a partner of the CARE institute, the LDC will continue to drive the commercialisation of biological medical products, known as biologicals, which emerge from stem cell research. The LDC and the CARE institute would like to intensify their cooperation particularly with small and medium-sized companies in the Munich area.
The CARE institute thereby aims to become a world-leading center for research in the area of regenerative medicine and a central focal point for scientists, developers, companies and medical staff. The development of new drugs and treatments at the center will not only benefit patients around the world but will also enhance Germany's position as a business location.
MT

