Exploding seed pods
A mathematical model explains how popping cress catapults its seeds into the air
Plants use many strategies to disperse their seeds, but among the most fascinating are exploding seed pods. Scientists had assumed that the energy to power these explosions was generated through the seed pods deforming as they dried out, but in the case of ‘popping cress’ (Cardamine hirsuta) this turns out not to be so. Scientists at the the Max Planck Institute for Plant Breeding Research in Cologne, Germany, found out that these seed pods don’t wait to dry before they explode.
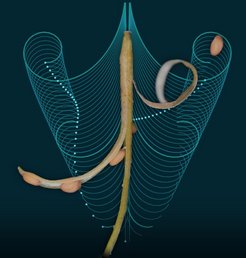
Since plants do not have muscles; rapid movements, like the exploding seed pods of popping cress, are rare in the plant kingdom. Scientists from different disciplines, led by Angela Hay, a plant geneticist at the Max Planck Institute in Cologne, worked together to discover how the seed pods of popping cress explode.
Explosive shatter of these seed pods is so fast that advanced high-speed cameras are needed to even see the explosion. Richard Bomphrey, of the Royal Veterinary College at the University of London, explains: “Because the seeds are so small, aerodynamic drag slows them down immediately.” To compensate, the seeds are accelerated away from the fruit and get up-to-speed extremely quickly. In fact, they accelerate from zero to ten metres per second in about half a millisecond.
Cells contract
Hay’s teams of scientists discovered that the secret to explosive acceleration in popping cress is the evolutionary innovation of a fruit wall that can store elastic energy through growth and expansion, and can rapidly release this energy at the right stage of development. Previously, scientists had claimed that tension was generated by differential contraction of the inner and outer layers of the seed pod as it dried. So what puzzled the researchers was how popping cress pods exploded while green and hydrated, rather than brown and dry. Their surprising discovery was that hydrated cells in the outer layer of the seed pod actually used their internal pressure in order to contract and generate tension. The authors used a computational model of three-dimensional plant cells, to show that when these cells were pressurized, they expanded in depth while contracting in length, “like the way an air mattress expands in depth, when inflated, but contracts in width,” explains Richard Smith, a computer scientist at Max Planck Institute in Cologne.
Cell wall forms a hinge
Another unexpected finding was how this energy was released. The authors found that the fruit wall wanted to coil along its length to release tension, but it had a curved cross-section preventing this. “This geometric constraint is also found in a toy called a slap bracelet,” explains Derek Moulton, of the Mathematical Institute at the University of Oxford. In both the toy and the seed pod, the cross-section first has to flatten before the tension is suddenly released by coiling. Unexpectedly, this mechanism relies on a unique cell wall geometry in the seed pod. As Moulton explains, “This wall is shaped like a hinge, which can open,” causing the fruit wall to flatten in cross-section and explosively coil.
According to Hay, their most exciting discovery was the evolutionary novelty of this hinged cell wall. They had evidence from genetics and mathematical modeling that this hinge was needed for explosive pod shatter, “but the fact that we found this hinge only in plants with explosive seed dispersal was the smoking gun,” says Hay.
After working out how the seed pods of popping cress exploded, the scientists realised that this mechanism had evolved via tweaking the shape of already-existing cellular components. One implication of their findings is that other movements in plants that were previously attributed to passive contraction by drying, may in fact be active processes, “especially in green, hydrated tissues,” says Smith.
This study is an example of the potential of interdisciplinary research. The scientists built up a comprehensive picture of explosive seed dispersal by relating observations at the plant scale all the way down to the cellular and genetic scales, and systematically linking each scale. As Alain Goriely, of the Mathematical Institute at the University of Oxford, says, “this approach was only made possible by combining state-of-the-art modelling techniques with biophysical measurements and biological experiments.”
AH/HR